Tuesday Poster Session
Category: Esophagus
P3901 - Efficacy of Dupilumab on Facilitated Food Introduction in Eosinophilic Esophagitis
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio
- JS
Jonathan Spergel, MD, PhD
Children’s Hospital of Philadelphia, Perelman School of Medicine at University of Pennsylvania School of Medicine
Philadelphia, PA
Presenting Author(s)
Award: Presidential Poster Award
NIcole Wolfset, MD1, Amanda Miur, MD1, Terri Brown-Whitehorn, MD2, Melanie A. Ruffner, MD, PhD3, Danielle Williams, MBA4, Sharon Carbonara, MS, BSN, RN5, Stephanie Marko, BSN, RN1, Susan Lee, 3, Matthew Ryan, MD1, Alain J. Benitez, MD6, Tiffany Pela, PharmD, MPH7, Amr Radwan, MA, MBBCh8, Sandy Durrani, MD9, Sabina deMarchi, PharmD8, Juby A. Jacob-Nara, MBBS, MD, MPH, MBA7, Jonathan M. Spergel, MD, PhD10
1Children's Hospital, Philadelphia, PA; 2Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA; 3Children’s Hospital of Philadelphia, Philadelphia, PA; 4CHOP, Philadelphia, PA; 5The Children’s Hospital of Philadelphia, Philadelphia, PA; 6Children's Hospital of Philadelphia, Philadelphia, PA; 7Sanofi, Bridgewater, NJ; 8Regeneron Pharmaceuticals Inc., Tarrytown, NY; 9Regeneron Pharmaceuticals Inc., Sleepy Hollow, NY; 10Children’s Hospital of Philadelphia, Perelman School of Medicine at University of Pennsylvania School of Medicine, Philadelphia, PA
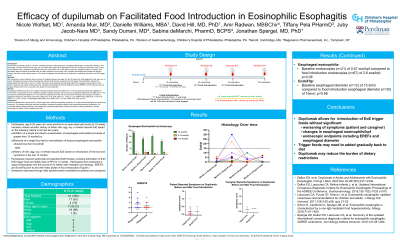
Introduction: Eosinophilic esophagitis (EoE) is a chronic, debilitating disease characterized by esophageal inflammation, eosinophilic infiltration, and dysphagia. Common foods like milk, egg, soy, and wheat often trigger EoE. Treatments include dietary avoidance, dupilumab, and off-label use of swallowed steroids or proton-pump-inhibitors. However, food elimination diets are difficult for patients and can affect adherence and quality of life. Patients and families seek simple and effective therapy that offer broader dietary options. This study investigates the potential role of dupilumab in facilitating introduction of EoE trigger foods.
Methods: We conducted an open label pilot study involving 21 patients between the ages of 6 and 25 years with EoE triggered by milk, egg, soy, or wheat. Participants received dupilumab alongside standard EoE therapy and elimination of their specific EoE trigger food, followed by standardized food introduction over 12 months. Symptoms were assessed through daily patient and caregiver questionnaires. Each patient underwent four upper endoscopies with evaluation by histology, Endoscopy Reference Score (EREFS), and Functional Luminal Imaging Probe (FLIP) prior to and with every phase of food introduction.
Results: No significant difference was found in patient reported symptom scores pre (SEM 0.07, n=16) and post food introduction (SEM 0.07, n=23) while on dupilumab (p=0.73). Similarly, caregiver reported symptom scores showed no significant difference pre (SEM 0.06, n=16) and post food introduction (SEM 0.07, n=23) while on dupilumab (p=0.36). Pre and post-food introduction endoscopies revealed no significant changes in eosinophil counts (Pre: SEM 0.40, n=18; Post: SEM 1.11, n=36; p= 0.10), EREFS (Pre: SEM 0.24, n=18; Post: SEM 0.11, n=36 ; p=0.09), or esophageal distensibility (Pre: SEM 0.45, n=12; Post: SEM 0.56, n=28; p=0.43).
Discussion: Dupilumab facilitated the reintroduction of EoE trigger foods without exacerbating symptoms or compromising histological and endoscopic outcomes. This therapy effectively controlled symptoms, preserved histologic integrity, and prevented endoscopic progression. By controlling disease while permitting a more liberal diet, dupilumab has the potential to reduce the burden of dietary restrictions, thereby enhancing the quality of life for patients with EoE.
Disclosures:
NIcole Wolfset, MD1, Amanda Miur, MD1, Terri Brown-Whitehorn, MD2, Melanie A. Ruffner, MD, PhD3, Danielle Williams, MBA4, Sharon Carbonara, MS, BSN, RN5, Stephanie Marko, BSN, RN1, Susan Lee, 3, Matthew Ryan, MD1, Alain J. Benitez, MD6, Tiffany Pela, PharmD, MPH7, Amr Radwan, MA, MBBCh8, Sandy Durrani, MD9, Sabina deMarchi, PharmD8, Juby A. Jacob-Nara, MBBS, MD, MPH, MBA7, Jonathan M. Spergel, MD, PhD10. P3901 - Efficacy of Dupilumab on Facilitated Food Introduction in Eosinophilic Esophagitis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
NIcole Wolfset, MD1, Amanda Miur, MD1, Terri Brown-Whitehorn, MD2, Melanie A. Ruffner, MD, PhD3, Danielle Williams, MBA4, Sharon Carbonara, MS, BSN, RN5, Stephanie Marko, BSN, RN1, Susan Lee, 3, Matthew Ryan, MD1, Alain J. Benitez, MD6, Tiffany Pela, PharmD, MPH7, Amr Radwan, MA, MBBCh8, Sandy Durrani, MD9, Sabina deMarchi, PharmD8, Juby A. Jacob-Nara, MBBS, MD, MPH, MBA7, Jonathan M. Spergel, MD, PhD10
1Children's Hospital, Philadelphia, PA; 2Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA; 3Children’s Hospital of Philadelphia, Philadelphia, PA; 4CHOP, Philadelphia, PA; 5The Children’s Hospital of Philadelphia, Philadelphia, PA; 6Children's Hospital of Philadelphia, Philadelphia, PA; 7Sanofi, Bridgewater, NJ; 8Regeneron Pharmaceuticals Inc., Tarrytown, NY; 9Regeneron Pharmaceuticals Inc., Sleepy Hollow, NY; 10Children’s Hospital of Philadelphia, Perelman School of Medicine at University of Pennsylvania School of Medicine, Philadelphia, PA
Introduction: Eosinophilic esophagitis (EoE) is a chronic, debilitating disease characterized by esophageal inflammation, eosinophilic infiltration, and dysphagia. Common foods like milk, egg, soy, and wheat often trigger EoE. Treatments include dietary avoidance, dupilumab, and off-label use of swallowed steroids or proton-pump-inhibitors. However, food elimination diets are difficult for patients and can affect adherence and quality of life. Patients and families seek simple and effective therapy that offer broader dietary options. This study investigates the potential role of dupilumab in facilitating introduction of EoE trigger foods.
Methods: We conducted an open label pilot study involving 21 patients between the ages of 6 and 25 years with EoE triggered by milk, egg, soy, or wheat. Participants received dupilumab alongside standard EoE therapy and elimination of their specific EoE trigger food, followed by standardized food introduction over 12 months. Symptoms were assessed through daily patient and caregiver questionnaires. Each patient underwent four upper endoscopies with evaluation by histology, Endoscopy Reference Score (EREFS), and Functional Luminal Imaging Probe (FLIP) prior to and with every phase of food introduction.
Results: No significant difference was found in patient reported symptom scores pre (SEM 0.07, n=16) and post food introduction (SEM 0.07, n=23) while on dupilumab (p=0.73). Similarly, caregiver reported symptom scores showed no significant difference pre (SEM 0.06, n=16) and post food introduction (SEM 0.07, n=23) while on dupilumab (p=0.36). Pre and post-food introduction endoscopies revealed no significant changes in eosinophil counts (Pre: SEM 0.40, n=18; Post: SEM 1.11, n=36; p= 0.10), EREFS (Pre: SEM 0.24, n=18; Post: SEM 0.11, n=36 ; p=0.09), or esophageal distensibility (Pre: SEM 0.45, n=12; Post: SEM 0.56, n=28; p=0.43).
Discussion: Dupilumab facilitated the reintroduction of EoE trigger foods without exacerbating symptoms or compromising histological and endoscopic outcomes. This therapy effectively controlled symptoms, preserved histologic integrity, and prevented endoscopic progression. By controlling disease while permitting a more liberal diet, dupilumab has the potential to reduce the burden of dietary restrictions, thereby enhancing the quality of life for patients with EoE.
Disclosures:
NIcole Wolfset indicated no relevant financial relationships.
Amanda Miur indicated no relevant financial relationships.
Terri Brown-Whitehorn: DBV technologies – Advisory Committee/Board Member, Consultant, Grant/Research Support. FARE – Grant/Research Support. NIH – Grant/Research Support. Regeneron – Grant/Research Support.
Melanie Ruffner indicated no relevant financial relationships.
Danielle Williams indicated no relevant financial relationships.
Sharon Carbonara indicated no relevant financial relationships.
Stephanie Marko indicated no relevant financial relationships.
Susan Lee indicated no relevant financial relationships.
Matthew Ryan indicated no relevant financial relationships.
Alain Benitez indicated no relevant financial relationships.
Tiffany Pela: Sanofi – Employee, Stock Options.
Amr Radwan: Regeneron Pharmaceuticals Inc. – Employee, Stock-publicly held company(excluding mutual/index funds).
Sandy Durrani: Regeneron Pharmaceuticals – Employee.
Sabina deMarchi: Regeneron Pharmaceuticals – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Juby A. Jacob-Nara: Sanofi – Employee, Stock Options.
Jonathan M. Spergel: Allakos – Consultant. DBV Technologies – Consultant, Grant/Research Support. Novartis – Consultant. Regeneron Pharmaceuticals Inc. – Consultant, Grant/Research Support. Shire/Takeda – Consultant.
NIcole Wolfset, MD1, Amanda Miur, MD1, Terri Brown-Whitehorn, MD2, Melanie A. Ruffner, MD, PhD3, Danielle Williams, MBA4, Sharon Carbonara, MS, BSN, RN5, Stephanie Marko, BSN, RN1, Susan Lee, 3, Matthew Ryan, MD1, Alain J. Benitez, MD6, Tiffany Pela, PharmD, MPH7, Amr Radwan, MA, MBBCh8, Sandy Durrani, MD9, Sabina deMarchi, PharmD8, Juby A. Jacob-Nara, MBBS, MD, MPH, MBA7, Jonathan M. Spergel, MD, PhD10. P3901 - Efficacy of Dupilumab on Facilitated Food Introduction in Eosinophilic Esophagitis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.

