Tuesday Poster Session
Category: Esophagus
P3924 - Risk-Aligned Management Guided by the Tissue Systems Pathology Test Can Improve Health Outcomes in Barrett’s Esophagus and Reduce Healthcare-Associated Costs
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

Has Audio
- CL
Cadman Leggett, MD
Mayo Clinic
Rochester, MN
Presenting Author(s)
Award: Presidential Poster Award
Cadman Leggett, MD1, Ananya Das, MD2, Amrit K.. Kamboj, MD3, Christian M. Smolko, PhD4, Meenakshi Arora, PhD4, Emmanuel C. Gorospe, MD, MPH, FACG5, Rebecca J. Critchley-Thorne, PhD4, Venkataraman R. Muthusamy, MD6
1Mayo Clinic, Rochester, MN; 2Arizona Centers for Digestive Health, Paradise Valley, AZ; 3Cedars-Sinai Medical Center, Los Angeles, CA; 4Castle Biosciences, Pittsburgh, PA; 5Castle Biosciences, Friendswood, TX; 6David Geffen School of Medicine at UCLA, Sherman Oaks, CA
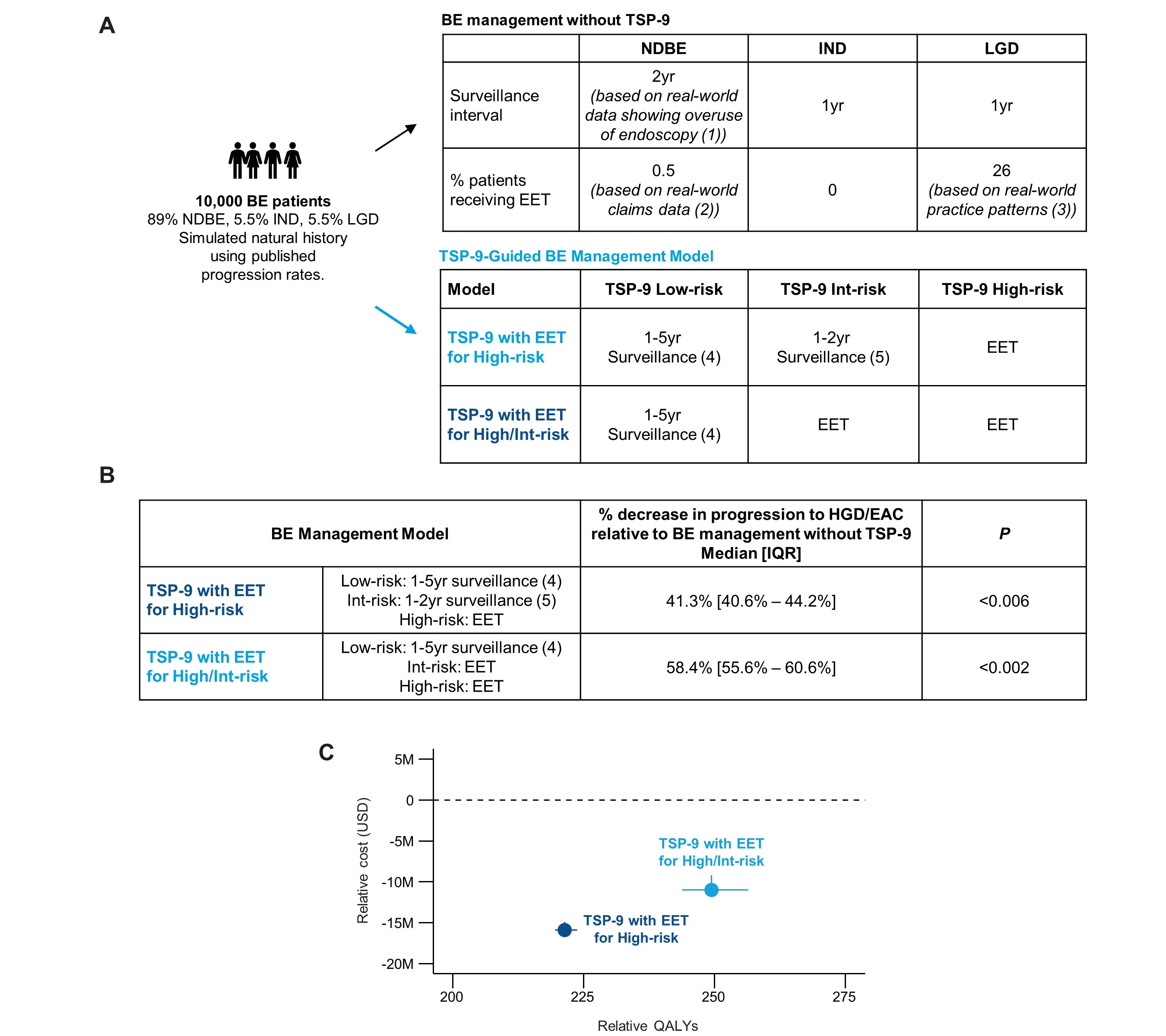
Introduction: The tissue systems pathology test (TissueCypher, TSP-9) has been validated to risk stratify patients with Barrett’s esophagus (BE) for progression to high-grade dysplasia (HGD) and esophageal adenocarcinoma (EAC). Studies have shown the utility of TSP-9 to select endoscopic eradication therapy (EET) or short-interval surveillance for patients at high- or intermediate-risk for progression and to attenuate surveillance frequency in low-risk patients. This study evaluated the cost-effectiveness of TSP-9-guided management for patients with BE.
Methods: A Markov decision and microsimulation model was used to generate a 5-year natural history of BE progression to HGD/EAC (Fig. 1A). Intervention models with surveillance intervals and EET were used to compare the cost-effectiveness of BE management based on literature and traditional guidelines vs. management guided by TSP-9 low-, intermediate (int)-, and high-risk results from the commercial payor perspective. Five-year health outcomes, costs, quality-adjusted life years (QALYs), and incremental cost-effectiveness ratios (ICERs) were evaluated.
Results: TSP-9-guided BE management significantly reduced HGD/EAC incidence by 41.3% (P < 0.006) and 58.4% (P < 0.002) when TSP-9 high-risk and high/int-risk patients received EET, respectively (Fig. 1B). ICERs for TSP-9-guided management relative to management without TSP-9 were -$71,900/QALY and -$43,942/QALY when high-risk and high/int-risk patients received EET, respectively, indicating TSP-9 is superior and should be adopted (Fig. 1C, Table 1). Cumulative BE management costs for 10,000 patients were reduced from $159M (IQR, $155-$162) or $2.11 per member per month (PMPM) for management without TSP-9 to $142M (IQR, $139-$145, P< 0.002) or $1.89 PMPM when TSP-9 high-risk patients (8.9%) received EET, and to $147M (IQR, $145-$149) or $1.95 PMPM when high/int-risk patients (21.4%) received EET, and low-risk patients (78.7%) were surveilled in 1-5 years based on the baseline pathology diagnosis (Table 1).
Discussion: TSP-9 enables risk-aligned management that targets highly effective EET and short-interval surveillance in higher risk patients with BE, leading to a significant reduction in HGD/EAC incidence. TSP-9 can reduce overuse of EET and surveillance in low-risk patients. By escalating care for higher risk patients and de-escalating care for low-risk patients, TSP-9 can improve health outcomes for patients with BE in a manner that is potentially cost-saving to the healthcare system.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Cadman Leggett, MD1, Ananya Das, MD2, Amrit K.. Kamboj, MD3, Christian M. Smolko, PhD4, Meenakshi Arora, PhD4, Emmanuel C. Gorospe, MD, MPH, FACG5, Rebecca J. Critchley-Thorne, PhD4, Venkataraman R. Muthusamy, MD6. P3924 - Risk-Aligned Management Guided by the Tissue Systems Pathology Test Can Improve Health Outcomes in Barrett’s Esophagus and Reduce Healthcare-Associated Costs, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
Cadman Leggett, MD1, Ananya Das, MD2, Amrit K.. Kamboj, MD3, Christian M. Smolko, PhD4, Meenakshi Arora, PhD4, Emmanuel C. Gorospe, MD, MPH, FACG5, Rebecca J. Critchley-Thorne, PhD4, Venkataraman R. Muthusamy, MD6
1Mayo Clinic, Rochester, MN; 2Arizona Centers for Digestive Health, Paradise Valley, AZ; 3Cedars-Sinai Medical Center, Los Angeles, CA; 4Castle Biosciences, Pittsburgh, PA; 5Castle Biosciences, Friendswood, TX; 6David Geffen School of Medicine at UCLA, Sherman Oaks, CA
Introduction: The tissue systems pathology test (TissueCypher, TSP-9) has been validated to risk stratify patients with Barrett’s esophagus (BE) for progression to high-grade dysplasia (HGD) and esophageal adenocarcinoma (EAC). Studies have shown the utility of TSP-9 to select endoscopic eradication therapy (EET) or short-interval surveillance for patients at high- or intermediate-risk for progression and to attenuate surveillance frequency in low-risk patients. This study evaluated the cost-effectiveness of TSP-9-guided management for patients with BE.
Methods: A Markov decision and microsimulation model was used to generate a 5-year natural history of BE progression to HGD/EAC (Fig. 1A). Intervention models with surveillance intervals and EET were used to compare the cost-effectiveness of BE management based on literature and traditional guidelines vs. management guided by TSP-9 low-, intermediate (int)-, and high-risk results from the commercial payor perspective. Five-year health outcomes, costs, quality-adjusted life years (QALYs), and incremental cost-effectiveness ratios (ICERs) were evaluated.
Results: TSP-9-guided BE management significantly reduced HGD/EAC incidence by 41.3% (P < 0.006) and 58.4% (P < 0.002) when TSP-9 high-risk and high/int-risk patients received EET, respectively (Fig. 1B). ICERs for TSP-9-guided management relative to management without TSP-9 were -$71,900/QALY and -$43,942/QALY when high-risk and high/int-risk patients received EET, respectively, indicating TSP-9 is superior and should be adopted (Fig. 1C, Table 1). Cumulative BE management costs for 10,000 patients were reduced from $159M (IQR, $155-$162) or $2.11 per member per month (PMPM) for management without TSP-9 to $142M (IQR, $139-$145, P< 0.002) or $1.89 PMPM when TSP-9 high-risk patients (8.9%) received EET, and to $147M (IQR, $145-$149) or $1.95 PMPM when high/int-risk patients (21.4%) received EET, and low-risk patients (78.7%) were surveilled in 1-5 years based on the baseline pathology diagnosis (Table 1).
Discussion: TSP-9 enables risk-aligned management that targets highly effective EET and short-interval surveillance in higher risk patients with BE, leading to a significant reduction in HGD/EAC incidence. TSP-9 can reduce overuse of EET and surveillance in low-risk patients. By escalating care for higher risk patients and de-escalating care for low-risk patients, TSP-9 can improve health outcomes for patients with BE in a manner that is potentially cost-saving to the healthcare system.

Figure: Figure 1: TSP-9-guided management can improve patient health outcomes without adding costs to the healthcare system. (A) BE management without TSP-9 model in which endoscopic surveillance intervals and use of EET were based on published literature showing real world endoscopy over-utilization, traditional guidelines, and commercial claims and encounters databases, and TSP-9-guided BE management model in which either all high- or all high/int-risk patients received EET and low-risk patients received endoscopic surveillance in 1-5 years depending on the baseline pathology diagnosis; (B) Percent progression to HGD/EAC for TSP-9-guided management models relative to progression in BE management without TSP-9 during five-year simulation; (C) BE management costs and QALYs for TSP-9-guided care models relative to the BE management without TSP-9; 1. BE management without TSP-9 surveillance intervals were based on: Wani S et al. Over-Utilization of Repeat Upper Endoscopy in Patients with Non-dysplastic Barrett's Esophagus: A Quality Registry Study. Am J Gastroenterol 2019;114:1256–1264; 2. Use of EET for patients with NDBE was based on commercial claims data from Merative MarketScan Commercial Claims and Encounters and Medicare Supplemental Databases; 3. EET use in all patients diagnosed with LGD assuming a subset are downstaged to NDBE/IND upon second pathology review and supported by Singh M et al. Practice patterns among U.S. gastroenterologists regarding endoscopic management of Barrett's esophagus. Gastrointest Endosc. 2013 Nov;78(5):689-95. 4. 1yr surveillance interval for LGD, 3yr for IND, and 5yr for NDBE; 5.1yr surveillance interval for IND/LGD, 2yr for NDBE.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Cadman Leggett: Castle Biosciences – Consultant.
Ananya Das: Clear note Health – Consultant.
Amrit Kamboj: Castle Biosciences – Consultant.
Christian Smolko: Castle Biosciences – Employee, Stock-publicly held company(excluding mutual/index funds).
Meenakshi Arora: Castle Biosciences – Employee, Stock Options.
Emmanuel Gorospe: Castle Biosciences – Employee.
Rebecca J. Critchley-Thorne: Castle Biosciences – Employee, Intellectual Property/Patents, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Venkataraman Muthusamy: Boston Scientific – Consultant, Grant/Research Support. Capsovision – Stock Options, Stock-privately held company. Castle Biosciences – Consultant, Speakers Bureau. Endogastric Solutions – Advisory Committee/Board Member, Consultant, Speakers Bureau. Medtronic – Consultant. Pentax Medical – Consultant.
Cadman Leggett, MD1, Ananya Das, MD2, Amrit K.. Kamboj, MD3, Christian M. Smolko, PhD4, Meenakshi Arora, PhD4, Emmanuel C. Gorospe, MD, MPH, FACG5, Rebecca J. Critchley-Thorne, PhD4, Venkataraman R. Muthusamy, MD6. P3924 - Risk-Aligned Management Guided by the Tissue Systems Pathology Test Can Improve Health Outcomes in Barrett’s Esophagus and Reduce Healthcare-Associated Costs, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.

