Tuesday Poster Session
Category: Esophagus
P3949 - GLP-1 RA Use in Patients with T2DM is Associated with Lower Risk of Esophageal Cancer: A National Analysis
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio

Muhammed Ceesay, MD
Charleston Area Medical Center
Charleston, WV
Presenting Author(s)
Mark Ayoub, MD1, Carol Faris, MD2, Muhammed Ceesay, MD3, Harleen Chela, MD1, Ebubekir Daglilar, MD1, Roberta Hunter, MD1
1West Virginia University - Charleston Area Medical Center, Charleston, WV; 2CarePoint Health - Bayonne Medical Center, Charleston, WV; 3Charleston Area Medical Center, Charleston, WV
Introduction: Glucagon-like peptide-1 receptor agonists (GLP-1 RA), such as exenatide, liraglutide, and semaglutide, are becoming more popular in managing type 2 diabetes mellitus (T2DM), especially for their cardiovascular, renal, and metabolic benefits. Despite this, concerns linger over their potential link to malignant neoplasms like pancreatic and thyroid cancers, requiring more research to clarify their safety profiles. Additionally, evidence suggests GLP-1RAs may lower colorectal and pancreatic cancer risk, especially in obese or overweight individuals, indicating a protective effect beyond weight loss. Current studies leave a gap in comprehensively understanding cancer risks associated with GLP-1RA which prompts further research to enhance our understanding of their overall safety.
Methods: We queried the US Collaborative Network (63 healthcare organizations) of TriNetX research database. Patients with T2DM were identified and divided into two cohorts; patients on GLP-1 RAs, and patients not on GLP-1 RAs. We excluded obese patients with body mass index (BMI) >25 kg/m2, family history of gastrointestinal malignancy, infectious mononucleosis, chronic gastritis, pernicious anemia, helicobacter pylori infection, and gastroesophageal reflux disease (GERD). We used a 1:1 propensity-score matching (PSM) model using patient’s baseline characteristics, medications, and genetics. A full list of PSM components is highlighted in table 1. We compared the rate of esophageal cancer at the 7-year mark.
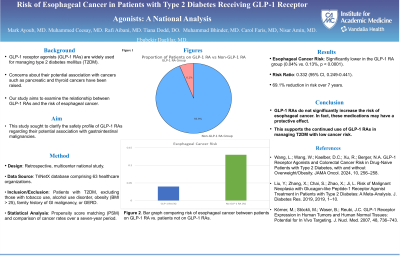
Results: A total of 3,851,241 patients with T2DM were identified. Of those, 7.7% (n=294,781) were on a GLP-1 RA and 92.3% (3,556,462) were not on a GLP-1 RA. After PSM, both cohorts included 294,779 patients. Patients with T2DM who were on a GLP-1 RA compared to those who are not had a statistically significant lower risk of esophageal cancer (0.04% vs 0.12%, p< 0.0001) at the 7-year mark. Esophageal cancer risk difference with GLP-1 RA use was -0.08% with a 95% CI (-0.09, -0.06).
Discussion: The use of GLP-1 RA in patients with T2DM is significantly associated with a lower risk of having esophageal cancer. This finding supports the continued use of GLP-1 analogues as a therapeutic option in managing T2DM, considering their well-established benefits and low risk of complications. Based on the study results, these medications may even have a protective effect.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Mark Ayoub, MD1, Carol Faris, MD2, Muhammed Ceesay, MD3, Harleen Chela, MD1, Ebubekir Daglilar, MD1, Roberta Hunter, MD1. P3949 - GLP-1 RA Use in Patients with T2DM is Associated with Lower Risk of Esophageal Cancer: A National Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1West Virginia University - Charleston Area Medical Center, Charleston, WV; 2CarePoint Health - Bayonne Medical Center, Charleston, WV; 3Charleston Area Medical Center, Charleston, WV
Introduction: Glucagon-like peptide-1 receptor agonists (GLP-1 RA), such as exenatide, liraglutide, and semaglutide, are becoming more popular in managing type 2 diabetes mellitus (T2DM), especially for their cardiovascular, renal, and metabolic benefits. Despite this, concerns linger over their potential link to malignant neoplasms like pancreatic and thyroid cancers, requiring more research to clarify their safety profiles. Additionally, evidence suggests GLP-1RAs may lower colorectal and pancreatic cancer risk, especially in obese or overweight individuals, indicating a protective effect beyond weight loss. Current studies leave a gap in comprehensively understanding cancer risks associated with GLP-1RA which prompts further research to enhance our understanding of their overall safety.
Methods: We queried the US Collaborative Network (63 healthcare organizations) of TriNetX research database. Patients with T2DM were identified and divided into two cohorts; patients on GLP-1 RAs, and patients not on GLP-1 RAs. We excluded obese patients with body mass index (BMI) >25 kg/m2, family history of gastrointestinal malignancy, infectious mononucleosis, chronic gastritis, pernicious anemia, helicobacter pylori infection, and gastroesophageal reflux disease (GERD). We used a 1:1 propensity-score matching (PSM) model using patient’s baseline characteristics, medications, and genetics. A full list of PSM components is highlighted in table 1. We compared the rate of esophageal cancer at the 7-year mark.
Results: A total of 3,851,241 patients with T2DM were identified. Of those, 7.7% (n=294,781) were on a GLP-1 RA and 92.3% (3,556,462) were not on a GLP-1 RA. After PSM, both cohorts included 294,779 patients. Patients with T2DM who were on a GLP-1 RA compared to those who are not had a statistically significant lower risk of esophageal cancer (0.04% vs 0.12%, p< 0.0001) at the 7-year mark. Esophageal cancer risk difference with GLP-1 RA use was -0.08% with a 95% CI (-0.09, -0.06).
Discussion: The use of GLP-1 RA in patients with T2DM is significantly associated with a lower risk of having esophageal cancer. This finding supports the continued use of GLP-1 analogues as a therapeutic option in managing T2DM, considering their well-established benefits and low risk of complications. Based on the study results, these medications may even have a protective effect.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Mark Ayoub indicated no relevant financial relationships.
Carol Faris indicated no relevant financial relationships.
Muhammed Ceesay indicated no relevant financial relationships.
Harleen Chela indicated no relevant financial relationships.
Ebubekir Daglilar indicated no relevant financial relationships.
Roberta Hunter indicated no relevant financial relationships.
Mark Ayoub, MD1, Carol Faris, MD2, Muhammed Ceesay, MD3, Harleen Chela, MD1, Ebubekir Daglilar, MD1, Roberta Hunter, MD1. P3949 - GLP-1 RA Use in Patients with T2DM is Associated with Lower Risk of Esophageal Cancer: A National Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
