Monday Poster Session
Category: Liver
P3153 - Ribavirin Treatment in Acute HEV-Induced Liver Injury in a Healthy US Female: A Case Report
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio

Nadera Altork, MD
MedStar Health-Georgetown/Washington Hospital Center
Washington, DC
Presenting Author(s)
Nadera Altork, MD1, Michele Fischer, MD2, Lukman Cheraghvandi, MD2, Arul M. Thomas, MD3
1MedStar Health-Georgetown/Washington Hospital Center, Washington, DC; 2MedStar Georgetown University Hospital, Washington, DC; 3Medstar Georgetown University Hospital, Washington, DC
Introduction: Hepatitis E virus (HEV) is an important cause of acute viral hepatitis globally. According to the U.S. Centers for Disease Control and Prevention (CDC), an estimated 20 million people contract HEV annually, resulting in approximately 3.3 million symptomatic cases and around 70,000 deaths globally. The largest outbreaks have occurred in endemic areas of south and central Asia, Africa, and tropical East Asia. Notably, vulnerable groups such as pregnant women, the immunosuppressed, the elderly, and those with chronic liver diseases are at an increased risk of progressing to severe acute liver injury and acute liver failure. We present a case of acute liver injury from acute HEV infection in a healthy non-pregnant female from U.S.
Case Description/Methods: A 36-year-old U.S. female, non-pregnant with no known liver disease, developed nonspecific symptoms of nausea, vomiting, and abdominal pain after traveling to Indonesia. She was found to have acute liver injury with concern for acute liver failure. Her aspartate aminotransferase (AST) was 4157 U/L, alanine transaminase (ALT) was 4245 U/L, total bilirubin reached 12 mg/dl, INR 3.6, hepatitis A, B, and C viral panel were negative, autoimmune markers were negative, including (ANA) and anti-smooth muscle antibody. She began liver transplant evaluation. Her liver biopsy showed severe acute hepatocellular necrosis ( > 50%) with mixed inflammatory infiltrate. HEV PCR was positive (93,700,000 IU/ml). After a review of risks and benefits in a young female, Ribavirin treatment was promptly started and helped lead to symptom resolution and biomarker normalization in 1 month. She did not require emergency liver transplantation and made a full recovery.
Discussion: Acute HEV infection should be considered in appropriate patients with acute liver injury in the U.S., particularly with known exposure risks. Ribavirin is the standard treatment for chronic HEV infections. It typically entails a three-month regimen. However, there is no FDA- approved medication for acute HEV infections. Further study is warranted to assess the potential benefits of Ribavirin therapy, in order to prevent major morbidity and mortality caused by acute HEV infection.
Disclosures:
Nadera Altork, MD1, Michele Fischer, MD2, Lukman Cheraghvandi, MD2, Arul M. Thomas, MD3. P3153 - Ribavirin Treatment in Acute HEV-Induced Liver Injury in a Healthy US Female: A Case Report, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1MedStar Health-Georgetown/Washington Hospital Center, Washington, DC; 2MedStar Georgetown University Hospital, Washington, DC; 3Medstar Georgetown University Hospital, Washington, DC
Introduction: Hepatitis E virus (HEV) is an important cause of acute viral hepatitis globally. According to the U.S. Centers for Disease Control and Prevention (CDC), an estimated 20 million people contract HEV annually, resulting in approximately 3.3 million symptomatic cases and around 70,000 deaths globally. The largest outbreaks have occurred in endemic areas of south and central Asia, Africa, and tropical East Asia. Notably, vulnerable groups such as pregnant women, the immunosuppressed, the elderly, and those with chronic liver diseases are at an increased risk of progressing to severe acute liver injury and acute liver failure. We present a case of acute liver injury from acute HEV infection in a healthy non-pregnant female from U.S.
Case Description/Methods: A 36-year-old U.S. female, non-pregnant with no known liver disease, developed nonspecific symptoms of nausea, vomiting, and abdominal pain after traveling to Indonesia. She was found to have acute liver injury with concern for acute liver failure. Her aspartate aminotransferase (AST) was 4157 U/L, alanine transaminase (ALT) was 4245 U/L, total bilirubin reached 12 mg/dl, INR 3.6, hepatitis A, B, and C viral panel were negative, autoimmune markers were negative, including (ANA) and anti-smooth muscle antibody. She began liver transplant evaluation. Her liver biopsy showed severe acute hepatocellular necrosis ( > 50%) with mixed inflammatory infiltrate. HEV PCR was positive (93,700,000 IU/ml). After a review of risks and benefits in a young female, Ribavirin treatment was promptly started and helped lead to symptom resolution and biomarker normalization in 1 month. She did not require emergency liver transplantation and made a full recovery.
Discussion: Acute HEV infection should be considered in appropriate patients with acute liver injury in the U.S., particularly with known exposure risks. Ribavirin is the standard treatment for chronic HEV infections. It typically entails a three-month regimen. However, there is no FDA- approved medication for acute HEV infections. Further study is warranted to assess the potential benefits of Ribavirin therapy, in order to prevent major morbidity and mortality caused by acute HEV infection.

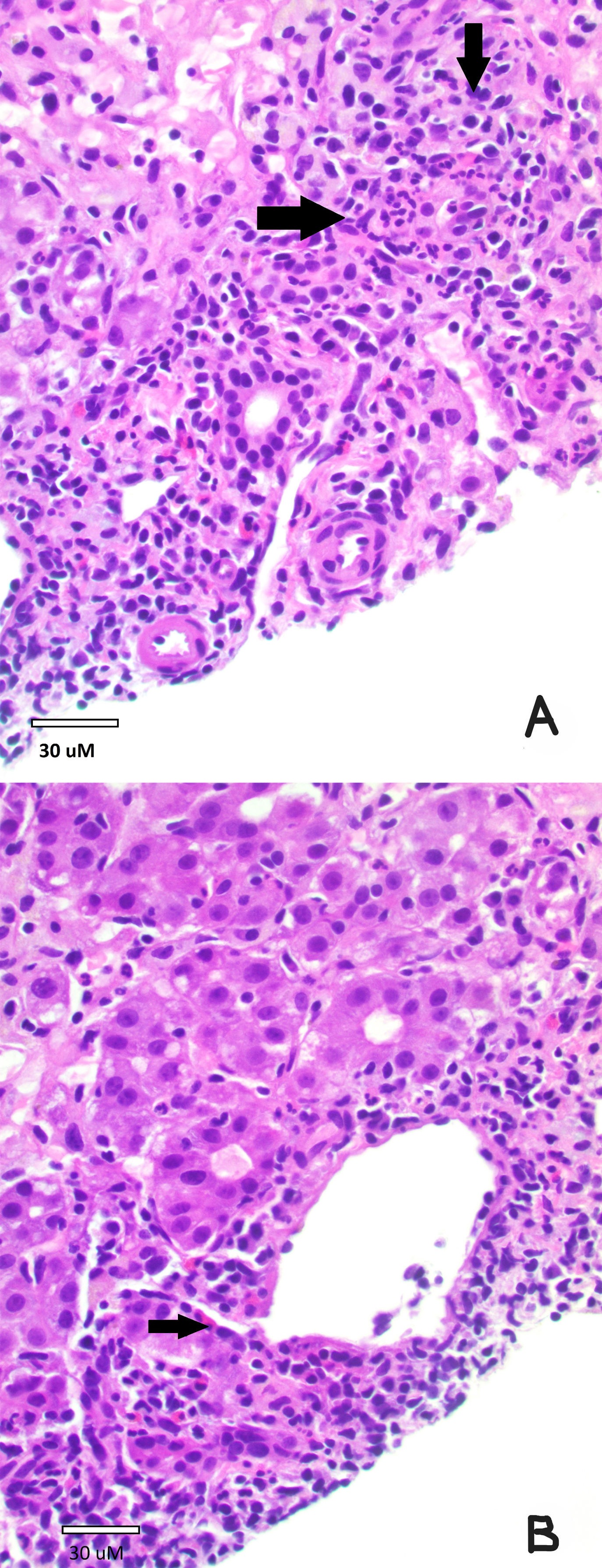
Figure: Liver Biopsy:
Image A: A portal tract with lymphoplasmacytic predominant interface hepatitis (both arrows). (H&E stain, 400x)
Image B: Central vein with severe mixed lymphoplasmacytic and eosinophilic (arrow) hepatitis. Also, pseudo-rosette formation of hepatocytes is noted. (H&E stain, 400x)
Image A: A portal tract with lymphoplasmacytic predominant interface hepatitis (both arrows). (H&E stain, 400x)
Image B: Central vein with severe mixed lymphoplasmacytic and eosinophilic (arrow) hepatitis. Also, pseudo-rosette formation of hepatocytes is noted. (H&E stain, 400x)
Disclosures:
Nadera Altork indicated no relevant financial relationships.
Michele Fischer indicated no relevant financial relationships.
Lukman Cheraghvandi indicated no relevant financial relationships.
Arul M. Thomas indicated no relevant financial relationships.
Nadera Altork, MD1, Michele Fischer, MD2, Lukman Cheraghvandi, MD2, Arul M. Thomas, MD3. P3153 - Ribavirin Treatment in Acute HEV-Induced Liver Injury in a Healthy US Female: A Case Report, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
