Monday Poster Session
Category: Liver
P3088 - Acute Autoimmune Hepatitis After COVID-19 Vaccine: Coincidence or Vaccine-Induced Phenomenon?
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio

Neelam Khetpal, MD
Hartford Hospital
Hartford, CT
Presenting Author(s)
Neelam Khetpal, MD1, Ranjeet Kumar, MD1, Fnu Partab, MBBS2
1Hartford Hospital, Hartford, CT; 2National Medical Center, Tampa, FL
Introduction: The COVID-19 pandemic has caused global morbidity and mortality. Vaccination, including mRNA-based and adenovirus vector-based, has emerged as a crucial strategy in mitigating the impact of COVID-19. Although effective, these vaccines are linked to myocarditis, vaccine-induced immune thrombotic thrombocytopenia, and autoimmune diseases. We present a rare case of autoimmune hepatitis (AIH) following COVID-19 vaccination, highlighting emerging concerns about vaccine-induced autoimmune diseases.
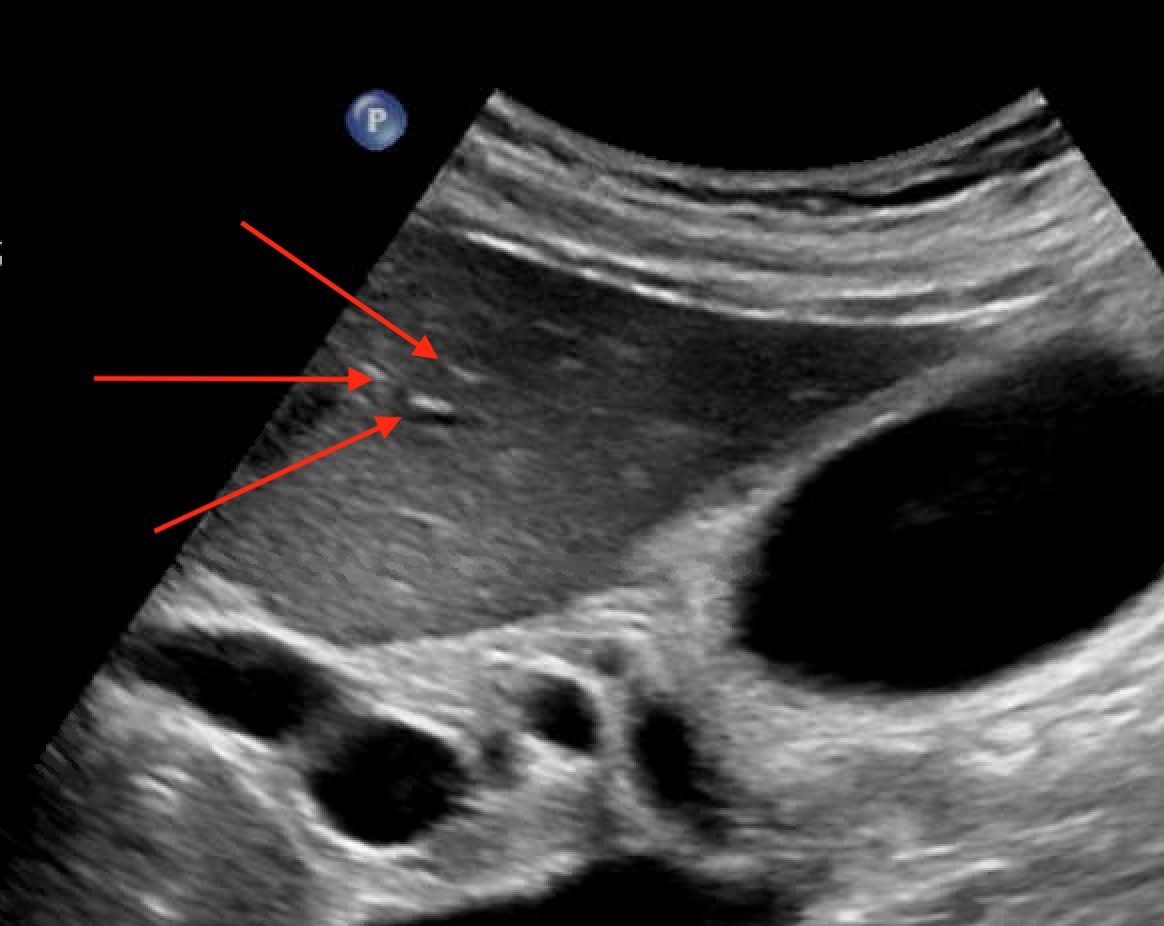
Case Description/Methods: A 57-year-old female with no significant medical history presented with jaundice and excessive fatigue for 2 weeks. She denied recent travel, medications, or alcohol use but had received a booster dose of the Pfizer COVID-19 vaccine just before the onset of symptoms. Examination showed scleral icterus. Blood work revealed elevated levels of bilirubin, AST, ALT, and ALP. Abdominal ultrasound showed a hepatic echotexture with a "starry sky" appearance, consistent with acute hepatitis (Figure 1). More workup revealed a positive ANA screen with a 1:1280 titer and cytoplasmic pattern. Anti-mitochondrial antibody was positive. Liver biopsy showed multiple portal tracts expanded by plasma cell-predominant inflammatory infiltrate with severe interface activity. The remaining hepatic parenchyma showed extensive lobular disarray with multiacinar collapse, apoptotic hepatocytes, and ceroid-containing macrophages. Overall, the findings are consistent with acute AIH. She was subsequently given IV methylprednisolone 40 mg daily, resulting in marked improvement (table 1). She was discharged with oral steroids and scheduled for hepatology follow-up.
Discussion: The presence of ceroid-containing macrophages and lobular disarray/hepatocytic necrosis, along with recent immunization, raises suspicion for drug-induced autoimmune hepatitis. AIH cases following COVID-19 vaccination range from 27 to 82 years of age, mostly in females, and occur after any vaccine dose. The time of symptom onset from vaccination varies from 1 day to 1 month, with jaundice occurring in nearly all cases. Abnormal LFTs are common, but normal results do not exclude AIH. Possible mechanisms include molecular mimicry, adjuvants, epitope spreading, bystander activation, X chromosome factors, and skeptical hepatotropism of SARS-CoV-2. Steroid therapy, with or without azathioprine, shows favorable outcomes. The key question is whether subsequent COVID-19 vaccine doses are safe when immune hepatitis develops post-vaccination
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Neelam Khetpal, MD1, Ranjeet Kumar, MD1, Fnu Partab, MBBS2. P3088 - Acute Autoimmune Hepatitis After COVID-19 Vaccine: Coincidence or Vaccine-Induced Phenomenon?, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Hartford Hospital, Hartford, CT; 2National Medical Center, Tampa, FL
Introduction: The COVID-19 pandemic has caused global morbidity and mortality. Vaccination, including mRNA-based and adenovirus vector-based, has emerged as a crucial strategy in mitigating the impact of COVID-19. Although effective, these vaccines are linked to myocarditis, vaccine-induced immune thrombotic thrombocytopenia, and autoimmune diseases. We present a rare case of autoimmune hepatitis (AIH) following COVID-19 vaccination, highlighting emerging concerns about vaccine-induced autoimmune diseases.
Case Description/Methods: A 57-year-old female with no significant medical history presented with jaundice and excessive fatigue for 2 weeks. She denied recent travel, medications, or alcohol use but had received a booster dose of the Pfizer COVID-19 vaccine just before the onset of symptoms. Examination showed scleral icterus. Blood work revealed elevated levels of bilirubin, AST, ALT, and ALP. Abdominal ultrasound showed a hepatic echotexture with a "starry sky" appearance, consistent with acute hepatitis (Figure 1). More workup revealed a positive ANA screen with a 1:1280 titer and cytoplasmic pattern. Anti-mitochondrial antibody was positive. Liver biopsy showed multiple portal tracts expanded by plasma cell-predominant inflammatory infiltrate with severe interface activity. The remaining hepatic parenchyma showed extensive lobular disarray with multiacinar collapse, apoptotic hepatocytes, and ceroid-containing macrophages. Overall, the findings are consistent with acute AIH. She was subsequently given IV methylprednisolone 40 mg daily, resulting in marked improvement (table 1). She was discharged with oral steroids and scheduled for hepatology follow-up.
Discussion: The presence of ceroid-containing macrophages and lobular disarray/hepatocytic necrosis, along with recent immunization, raises suspicion for drug-induced autoimmune hepatitis. AIH cases following COVID-19 vaccination range from 27 to 82 years of age, mostly in females, and occur after any vaccine dose. The time of symptom onset from vaccination varies from 1 day to 1 month, with jaundice occurring in nearly all cases. Abnormal LFTs are common, but normal results do not exclude AIH. Possible mechanisms include molecular mimicry, adjuvants, epitope spreading, bystander activation, X chromosome factors, and skeptical hepatotropism of SARS-CoV-2. Steroid therapy, with or without azathioprine, shows favorable outcomes. The key question is whether subsequent COVID-19 vaccine doses are safe when immune hepatitis develops post-vaccination

Figure: Figure 1: Hypoechoic echotexture of the liver with a "starry sky" (red arrows) appearance reflecting echogenic portal triads and portal venous walls.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Neelam Khetpal indicated no relevant financial relationships.
Ranjeet Kumar indicated no relevant financial relationships.
Fnu Partab indicated no relevant financial relationships.
Neelam Khetpal, MD1, Ranjeet Kumar, MD1, Fnu Partab, MBBS2. P3088 - Acute Autoimmune Hepatitis After COVID-19 Vaccine: Coincidence or Vaccine-Induced Phenomenon?, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
