Monday Poster Session
Category: Liver
P3092 - A Case of Drug-Induced Liver Injury Caused by Mushroom Gummy Ingestion
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

Has Audio

Ishan Antony, MD
Lahey Hospital and Medical Center
Burlington, MA
Presenting Author(s)
Ishan Antony, MD1, Adel Farhoud, MD2, Ming Valerie. Lin, MD1
1Lahey Hospital and Medical Center, Burlington, MA; 2Lahey Hospital and Medical Center, Manchester, NH
Introduction: Drug-induced liver injury (DILI) is used to describe any injury to the liver caused by medications or supplements. The diagnosis of DILI relies on the exclusion of other etiologies of liver disease as specific biomarkers are still lacking. We report a case of DILI from a mushroom gummy supplement which was treated successfully with N-acetylcysteine (NAC).
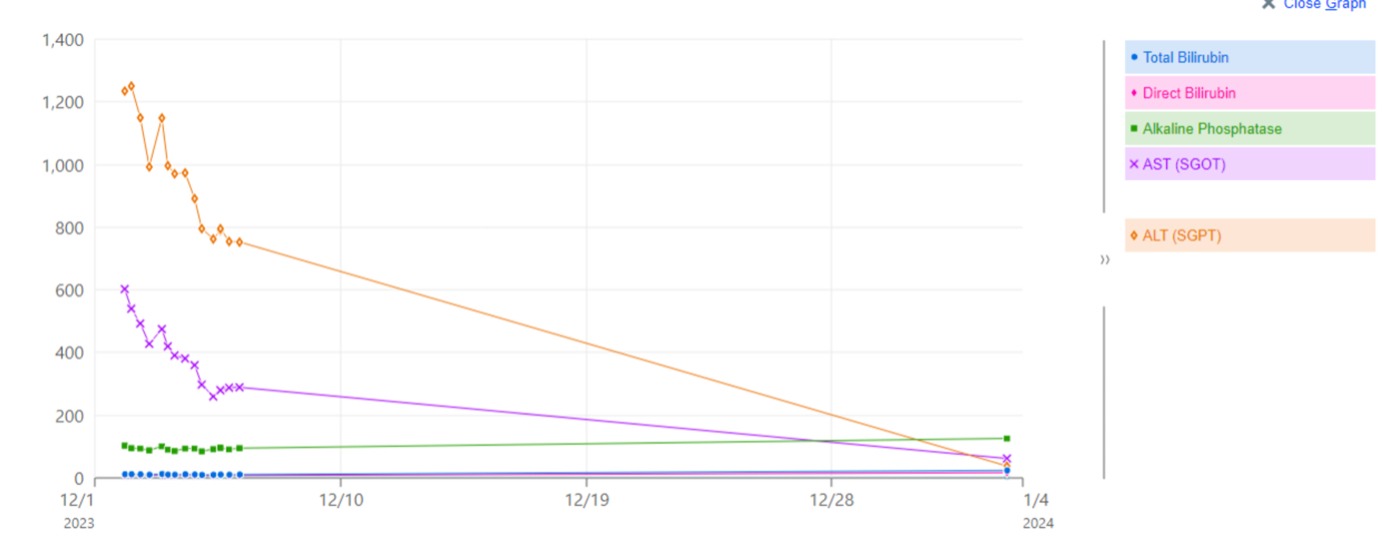
Case Description/Methods: A 32-year-old male with a history of alcohol use disorder and necrotizing pancreatitis presented to an outside hospital (OSH) with jaundice and pruritis over 2.5 weeks. He denied any history of liver disease or abnormal liver enzymes. He started a mushroom gummy last month. He reported his last alcohol drink was 5 years ago. He denied any history of blood transfusion, IV drug use, tattoos, new medications, or antibiotics. At the OSH, he was noted to have an elevated AST and ALT at 688 U/L and 1080 U/L respectively, and elevated T Bilirubin at 10.1 mg/dL. The patient was transferred 5 days later to our center for management. Table 1 lists all the drug, serological, and autoimmune workups done at admission. RUQUS showed mild CBD dilation. MRCP showed mild biliary ductal dilation with abrupt tapering at the pancreatic neck most likely due to an inflammatory stricture related to repetitive pancreatitis with no evidence of biliary obstruction. Poison control was contacted, and he was treated with NAC for 4 days with LFTs improving. Follow-up LFTs in a month showed normalization of AST, ALT, and T Bilirubin. Figure 1 notes the LFT trend from admission to our center till outpatient follow-up.
Discussion: Amanita phalloides have been widely noted as a cause of DILI but mushroom supplements have rarely been reported as a cause. This case reflects on the increasing DILI from nutritional supplements seen around the world. Approximately half the United States adult population consumes herbals and dietary supplements, and the proportion of supplement-related DILI cases has doubled from 7–9% in 2004–2007 to 19–20% in 2010–2014. The time to onset or latency of DILI varies and ranges between 5 days and 3 months, which correlates with the latency of our case. Time to recovery takes weeks to months after cessation of medication as seen in our case. The utilization of management of DILI with NAC in this case aligns with its current use with non-acetaminophen toxicity with data showing improved overall survival, transplant-free survival, and decreased length of hospital stay.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Ishan Antony, MD1, Adel Farhoud, MD2, Ming Valerie. Lin, MD1. P3092 - A Case of Drug-Induced Liver Injury Caused by Mushroom Gummy Ingestion, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Lahey Hospital and Medical Center, Burlington, MA; 2Lahey Hospital and Medical Center, Manchester, NH
Introduction: Drug-induced liver injury (DILI) is used to describe any injury to the liver caused by medications or supplements. The diagnosis of DILI relies on the exclusion of other etiologies of liver disease as specific biomarkers are still lacking. We report a case of DILI from a mushroom gummy supplement which was treated successfully with N-acetylcysteine (NAC).
Case Description/Methods: A 32-year-old male with a history of alcohol use disorder and necrotizing pancreatitis presented to an outside hospital (OSH) with jaundice and pruritis over 2.5 weeks. He denied any history of liver disease or abnormal liver enzymes. He started a mushroom gummy last month. He reported his last alcohol drink was 5 years ago. He denied any history of blood transfusion, IV drug use, tattoos, new medications, or antibiotics. At the OSH, he was noted to have an elevated AST and ALT at 688 U/L and 1080 U/L respectively, and elevated T Bilirubin at 10.1 mg/dL. The patient was transferred 5 days later to our center for management. Table 1 lists all the drug, serological, and autoimmune workups done at admission. RUQUS showed mild CBD dilation. MRCP showed mild biliary ductal dilation with abrupt tapering at the pancreatic neck most likely due to an inflammatory stricture related to repetitive pancreatitis with no evidence of biliary obstruction. Poison control was contacted, and he was treated with NAC for 4 days with LFTs improving. Follow-up LFTs in a month showed normalization of AST, ALT, and T Bilirubin. Figure 1 notes the LFT trend from admission to our center till outpatient follow-up.
Discussion: Amanita phalloides have been widely noted as a cause of DILI but mushroom supplements have rarely been reported as a cause. This case reflects on the increasing DILI from nutritional supplements seen around the world. Approximately half the United States adult population consumes herbals and dietary supplements, and the proportion of supplement-related DILI cases has doubled from 7–9% in 2004–2007 to 19–20% in 2010–2014. The time to onset or latency of DILI varies and ranges between 5 days and 3 months, which correlates with the latency of our case. Time to recovery takes weeks to months after cessation of medication as seen in our case. The utilization of management of DILI with NAC in this case aligns with its current use with non-acetaminophen toxicity with data showing improved overall survival, transplant-free survival, and decreased length of hospital stay.

Figure: Patient's progression of liver function tests during hospitalization. ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Ishan Antony indicated no relevant financial relationships.
Adel Farhoud indicated no relevant financial relationships.
Ming Lin indicated no relevant financial relationships.
Ishan Antony, MD1, Adel Farhoud, MD2, Ming Valerie. Lin, MD1. P3092 - A Case of Drug-Induced Liver Injury Caused by Mushroom Gummy Ingestion, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
