Monday Poster Session
Category: Liver
P3020 - Drug-Induced Liver Injury from Daptomycin: A Rare Case Report With Isolated Transaminitis
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio
- KE
Khaled Elsokary, DO
Albany Medical Center
Albany, NY
Presenting Author(s)
Khaled Elsokary, DO1, Osama Alshakhatreh, MD1, Ibrahim Mohammed, MD1, Mohamed Ismail, DO2, Maheep Sangha, MD1, Seth Richter, MD1
1Albany Medical Center, Albany, NY; 2Rutgers New Jersey Medical School, Newark, NJ
Introduction: Drug-induced liver injury (DILI) is a known complication of many medications, with over a thousand drugs associated with it. While various mechanisms have been identified, predicting liver interactions with drugs remains unpredictable. Daptomycin is rarely linked to liver injury, with only a few cases reported. Diagnosis can be challenging due to the varied ways it presents. Elevated liver enzymes, typically seen in conditions like acetaminophen toxicity or shock syndromes, are unusual in daptomycin-induced liver injury. This case report describes likely liver damage following intravenous daptomycin administration, without evidence of other complications like rhabdomyolysis or hyperbilirubinemia.
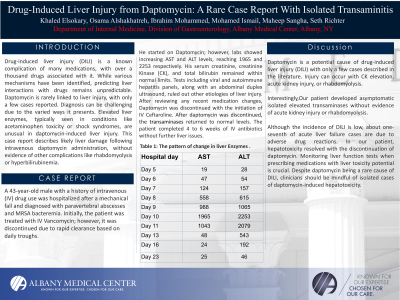
Case Description/Methods: A 43-year-old male with a history of intravenous (IV) drug use was hospitalized after a mechanical fall and diagnosed with paravertebral abscesses and MRSA bacteremia. Initially, the patient was treated with IV Vancomycin; however, it was discontinued due to rapid clearance based on daily troughs. He started on Daptomycin; however, labs showed increasing AST and ALT levels, reaching 1965 and 2253 respectively. His serum creatinine, creatinine Kinase (CK), and total bilirubin remained within normal limits. Tests including viral and autoimmune hepatitis panels, along with an abdominal duplex ultrasound, ruled out other etiologies of liver injury. After reviewing any recent medication changes, Daptomycin was discontinued with the initiation of IV Ceftaroline. After daptomycin was discontinued, the transaminases returned to normal levels. The patient completed 4 to 6 weeks of IV antibiotics without further liver issues.
Discussion: Daptomycin is a potential cause of drug-induced liver injury (DILI) with only a few cases described in the literature. Injury can occur with CK elevation, acute kidney injury, or rhabdomyolysis. Interestingly, our patient developed asymptomatic, isolated elevated transaminases without evidence of acute kidney injury or rhabdomyolysis.
Although the incidence of DILI is low, about one-seventh of acute liver failure cases are due to adverse drug reactions. In our patient, hepatotoxicity resolved with the discontinuation of daptomycin. Monitoring liver function tests when prescribing medications with liver toxicity potential is crucial. Despite daptomycin being a rare cause of DILI, clinicians should be mindful of isolated cases of daptomycin-induced hepatotoxicity.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Khaled Elsokary, DO1, Osama Alshakhatreh, MD1, Ibrahim Mohammed, MD1, Mohamed Ismail, DO2, Maheep Sangha, MD1, Seth Richter, MD1. P3020 - Drug-Induced Liver Injury from Daptomycin: A Rare Case Report With Isolated Transaminitis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Albany Medical Center, Albany, NY; 2Rutgers New Jersey Medical School, Newark, NJ
Introduction: Drug-induced liver injury (DILI) is a known complication of many medications, with over a thousand drugs associated with it. While various mechanisms have been identified, predicting liver interactions with drugs remains unpredictable. Daptomycin is rarely linked to liver injury, with only a few cases reported. Diagnosis can be challenging due to the varied ways it presents. Elevated liver enzymes, typically seen in conditions like acetaminophen toxicity or shock syndromes, are unusual in daptomycin-induced liver injury. This case report describes likely liver damage following intravenous daptomycin administration, without evidence of other complications like rhabdomyolysis or hyperbilirubinemia.
Case Description/Methods: A 43-year-old male with a history of intravenous (IV) drug use was hospitalized after a mechanical fall and diagnosed with paravertebral abscesses and MRSA bacteremia. Initially, the patient was treated with IV Vancomycin; however, it was discontinued due to rapid clearance based on daily troughs. He started on Daptomycin; however, labs showed increasing AST and ALT levels, reaching 1965 and 2253 respectively. His serum creatinine, creatinine Kinase (CK), and total bilirubin remained within normal limits. Tests including viral and autoimmune hepatitis panels, along with an abdominal duplex ultrasound, ruled out other etiologies of liver injury. After reviewing any recent medication changes, Daptomycin was discontinued with the initiation of IV Ceftaroline. After daptomycin was discontinued, the transaminases returned to normal levels. The patient completed 4 to 6 weeks of IV antibiotics without further liver issues.
Discussion: Daptomycin is a potential cause of drug-induced liver injury (DILI) with only a few cases described in the literature. Injury can occur with CK elevation, acute kidney injury, or rhabdomyolysis. Interestingly, our patient developed asymptomatic, isolated elevated transaminases without evidence of acute kidney injury or rhabdomyolysis.
Although the incidence of DILI is low, about one-seventh of acute liver failure cases are due to adverse drug reactions. In our patient, hepatotoxicity resolved with the discontinuation of daptomycin. Monitoring liver function tests when prescribing medications with liver toxicity potential is crucial. Despite daptomycin being a rare cause of DILI, clinicians should be mindful of isolated cases of daptomycin-induced hepatotoxicity.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Khaled Elsokary indicated no relevant financial relationships.
Osama Alshakhatreh indicated no relevant financial relationships.
Ibrahim Mohammed indicated no relevant financial relationships.
Mohamed Ismail indicated no relevant financial relationships.
Maheep Sangha indicated no relevant financial relationships.
Seth Richter indicated no relevant financial relationships.
Khaled Elsokary, DO1, Osama Alshakhatreh, MD1, Ibrahim Mohammed, MD1, Mohamed Ismail, DO2, Maheep Sangha, MD1, Seth Richter, MD1. P3020 - Drug-Induced Liver Injury from Daptomycin: A Rare Case Report With Isolated Transaminitis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
