Monday Poster Session
Category: Interventional Endoscopy
P2793 - Assessing the Risk of Post-ERCP Pancreatitis and other ERCP Complications Among Patients Given Prophylactic Oral Tacrolimus
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio
- SG
Sanya Goswami, MD
SUNY Downstate Health Sciences University
Brooklyn, NY
Presenting Author(s)
Sanya Goswami, MD1, Rahul Raiker, MD2, Haig Pakhchanian, MD3, Amar Manvar, MD4, Jessica Widmer, MD5
1SUNY Downstate Health Sciences University, Brooklyn, NY; 2George Washington University School of Medicine and Health Sciences, Washington, DC; 3RWJBarnabas Health, Toms River, NJ; 4NYU Langone Health, Mineola, NY; 5NYU Langone Health, New York, NY
Introduction: Post-ERCP pancreatitis (PEP) is the most common adverse event of endoscopic retrograde cholangiopancreatography (ERCP). In 2023, ASGE released guidelines recommending aggressive hydration, rectal NSAIDS, and prophylactic pancreatic stenting to prevent PEP. Experimental mice model studies have shown administration of rectal tacrolimus as a critical mediator of PEP. This study examines the risk of PEP and other ERCP complications among patients given oral tacrolimus versus rectal indomethacin or no prophylactic drug.
Methods: A retrospective cohort analysis was conducted using TriNetX, a multi-center database of ~110 million patients across 80 healthcare organizations. Adults who underwent an ERCP were identified from 2006-2024 and split among those who were only given prophylactic oral tacrolimus, rectal indomethacin, or neither prophylactic drug administered 1 day before or on the day of ERCP. 1:1 propensity score matching was performed, controlling for demographics and comorbidities, to assess 30-day adjusted risk ratios (aRR) for post-ERCP complications which included any episode of gastrointestinal (GI) bleed, PEP, GI perforation, ICU admission, sepsis, and all-cause mortality. Comparisons were conducted between oral tacrolimus versus no prophylactic drug and oral tacrolimus versus rectal indomethacin.
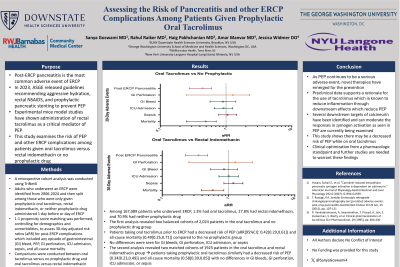
Results: Among 167,089 patients who underwent ERCP, 1.3% had oral tacrolimus, 27.8% had rectal indomethacin, and 70.9% had neither. The first analysis revealed two balanced cohorts of 2,024 patients in the oral tacrolimus and no prophylactic drug group. Patients taking oral tacrolimus prior to ERCP had a decreased risk of PEP (aRR[95%CI]: 0.42[0.29,0.61]) and all-cause mortality (0.49[0.25,0.71]) compared to the no prophylactic group. No differences were seen for GI bleeds, perforation, ICU admission, or sepsis. The second analysis revealed two matched cohorts of 1925 patients in the oral tacrolimus and rectal indomethacin group. Patients taking prophylactic oral tacrolimus similarly had a decreased risk of PEP (0.34[0.23,0.49]) and all-cause mortality (0.58[0.39,0.85]) with no differences in GI bleeds, perforation, ICU admission, or sepsis.
Discussion: As PEP remains a serious adverse event, novel therapies have emerged for prevention. Preclinical data supports a rationale for tacrolimus which is known to reduce inflammation with a downstream effect in reducing PEP. This study oral tacrolimus may decrease the risk of PEP. Further studies are warranted to validate these findings.
Disclosures:
Sanya Goswami, MD1, Rahul Raiker, MD2, Haig Pakhchanian, MD3, Amar Manvar, MD4, Jessica Widmer, MD5. P2793 - Assessing the Risk of Post-ERCP Pancreatitis and other ERCP Complications Among Patients Given Prophylactic Oral Tacrolimus, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1SUNY Downstate Health Sciences University, Brooklyn, NY; 2George Washington University School of Medicine and Health Sciences, Washington, DC; 3RWJBarnabas Health, Toms River, NJ; 4NYU Langone Health, Mineola, NY; 5NYU Langone Health, New York, NY
Introduction: Post-ERCP pancreatitis (PEP) is the most common adverse event of endoscopic retrograde cholangiopancreatography (ERCP). In 2023, ASGE released guidelines recommending aggressive hydration, rectal NSAIDS, and prophylactic pancreatic stenting to prevent PEP. Experimental mice model studies have shown administration of rectal tacrolimus as a critical mediator of PEP. This study examines the risk of PEP and other ERCP complications among patients given oral tacrolimus versus rectal indomethacin or no prophylactic drug.
Methods: A retrospective cohort analysis was conducted using TriNetX, a multi-center database of ~110 million patients across 80 healthcare organizations. Adults who underwent an ERCP were identified from 2006-2024 and split among those who were only given prophylactic oral tacrolimus, rectal indomethacin, or neither prophylactic drug administered 1 day before or on the day of ERCP. 1:1 propensity score matching was performed, controlling for demographics and comorbidities, to assess 30-day adjusted risk ratios (aRR) for post-ERCP complications which included any episode of gastrointestinal (GI) bleed, PEP, GI perforation, ICU admission, sepsis, and all-cause mortality. Comparisons were conducted between oral tacrolimus versus no prophylactic drug and oral tacrolimus versus rectal indomethacin.
Results: Among 167,089 patients who underwent ERCP, 1.3% had oral tacrolimus, 27.8% had rectal indomethacin, and 70.9% had neither. The first analysis revealed two balanced cohorts of 2,024 patients in the oral tacrolimus and no prophylactic drug group. Patients taking oral tacrolimus prior to ERCP had a decreased risk of PEP (aRR[95%CI]: 0.42[0.29,0.61]) and all-cause mortality (0.49[0.25,0.71]) compared to the no prophylactic group. No differences were seen for GI bleeds, perforation, ICU admission, or sepsis. The second analysis revealed two matched cohorts of 1925 patients in the oral tacrolimus and rectal indomethacin group. Patients taking prophylactic oral tacrolimus similarly had a decreased risk of PEP (0.34[0.23,0.49]) and all-cause mortality (0.58[0.39,0.85]) with no differences in GI bleeds, perforation, ICU admission, or sepsis.
Discussion: As PEP remains a serious adverse event, novel therapies have emerged for prevention. Preclinical data supports a rationale for tacrolimus which is known to reduce inflammation with a downstream effect in reducing PEP. This study oral tacrolimus may decrease the risk of PEP. Further studies are warranted to validate these findings.
Disclosures:
Sanya Goswami indicated no relevant financial relationships.
Rahul Raiker indicated no relevant financial relationships.
Haig Pakhchanian indicated no relevant financial relationships.
Amar Manvar indicated no relevant financial relationships.
Jessica Widmer indicated no relevant financial relationships.
Sanya Goswami, MD1, Rahul Raiker, MD2, Haig Pakhchanian, MD3, Amar Manvar, MD4, Jessica Widmer, MD5. P2793 - Assessing the Risk of Post-ERCP Pancreatitis and other ERCP Complications Among Patients Given Prophylactic Oral Tacrolimus, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
