Monday Poster Session
Category: IBD
P2635 - A New, Multifaceted STRIDE II-Based Monitoring Protocol for Advanced Therapy Starts in Inflammatory Bowel Disease
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

Has Audio

Scott MacKay, MD
University of Alberta
Edmonton, AB, Canada
Presenting Author(s)
Scott MacKay, MD, Denise Parsons, BA, Frank Hoentjen, MD, PhD, Levinus Dieleman, MD, PhD, Michal Gozdzik, MD, Karen Kroeker, MD, MSc, Karen Wong, MD, Todd McMullen, MD, Farhad Peerani, MD, Brendan Halloran, MD
University of Alberta, Edmonton, AB, Canada
Introduction: Outreach monitoring in inflammatory bowel disease (IBD) has been shown to be cost-effective and reduce healthcare utilization. The STRIDE-II guidelines recommend collection of patient-reported outcomes (PRO’s), biomarkers (e.g., fecal calprotectin (FCP), C-reactive protein (CRP)), and endoscopy to determine if patients with IBD are reaching defined therapeutic targets. The optimal way to apply these recommendations to new advanced therapies in IBD remains unclear. We designed a proactive, outreach monitoring protocol for new advanced therapy starts in IBD to assess clinical response and facilitate responsive disease management.
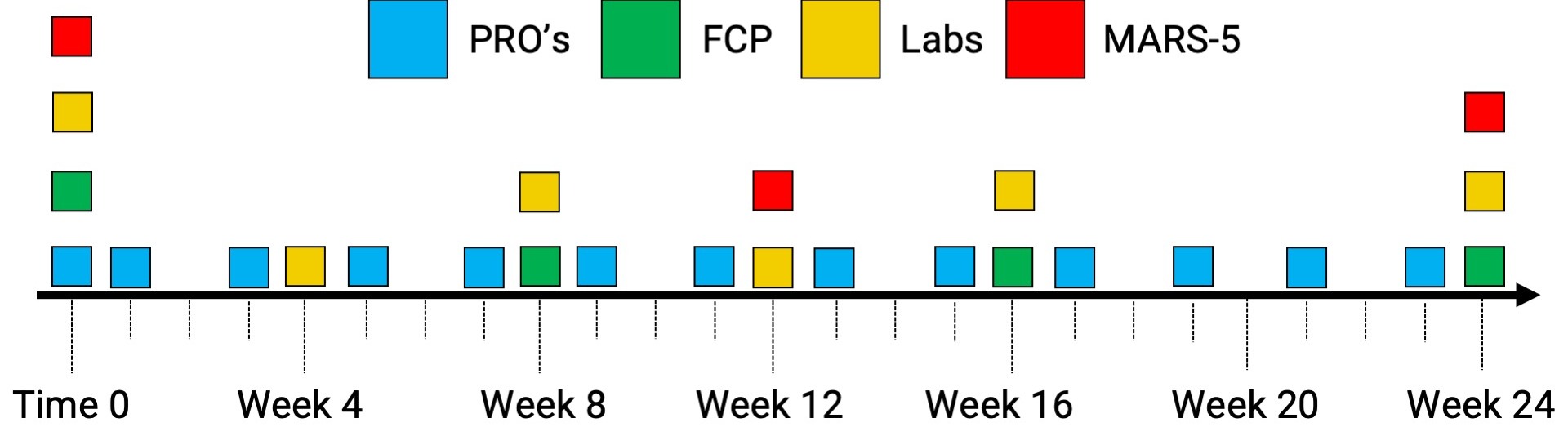
Methods: IBD patients complete 24 weeks of outreach monitoring after starting a new advanced therapy. PRO’s are collected on days 0, 3, and 7, and every 2 weeks thereafter. Serum labs are collected at baseline and weeks 4, 8, 12, 16, and 24 and FCP is collected at baseline and weeks 8, 16, and 24. Self-reported medication adherence is assessed at baseline and weeks 12 and 24. Endoscopy is booked 6-12 months from the start date. Granular clinical reports summarizing these results and the dates of last IBD review and last flare are sent to each treating gastroenterologist via our electronic medical record.
Results: 62 patients have been monitored with our protocol on the following biologic and small molecule therapies: ustekinumab (n = 30), tofacitinib (n = 14), risankizumab (n = 11), upacitinib (n = 4), vedolizumab (n = 2), and infliximab (n = 1). 41 patients (66.1%) had failed 1+ prior advanced therapies and 7 (11.3%) had prior IBD surgery. In 5 cases (8.1%), the patient was switched from their initial agent before 24 weeks due to lack of response. 54 patients (87.0%) complied with all FCP collections and 60 (96.8%) completed 95+% of PRO’s. Per STRIDE-II definitions, 38 patients (61.3%) demonstrated clinical response during the protocol and 20 (35.1%) achieved and maintained remission at 24 weeks. 14 patients (22.6%) received steroid courses, 9 (14.5%) presented to an emergency department, and 5 (8.1%) were hospitalized for their IBD during monitoring.
Discussion: Our STRIDE II based outreach monitoring protocol provided granular detail of clinical response to advanced IBD therapies to treating physicians. Patients were highly compliant with serial collection of PRO’s, FCP, and serum labs. This protocol allows for highly responsive disease management for IBD patients and may lead to treatment-specific monitoring recommendations and reduced healthcare utilization.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Scott MacKay, MD, Denise Parsons, BA, Frank Hoentjen, MD, PhD, Levinus Dieleman, MD, PhD, Michal Gozdzik, MD, Karen Kroeker, MD, MSc, Karen Wong, MD, Todd McMullen, MD, Farhad Peerani, MD, Brendan Halloran, MD. P2635 - A New, Multifaceted STRIDE II-Based Monitoring Protocol for Advanced Therapy Starts in Inflammatory Bowel Disease, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
University of Alberta, Edmonton, AB, Canada
Introduction: Outreach monitoring in inflammatory bowel disease (IBD) has been shown to be cost-effective and reduce healthcare utilization. The STRIDE-II guidelines recommend collection of patient-reported outcomes (PRO’s), biomarkers (e.g., fecal calprotectin (FCP), C-reactive protein (CRP)), and endoscopy to determine if patients with IBD are reaching defined therapeutic targets. The optimal way to apply these recommendations to new advanced therapies in IBD remains unclear. We designed a proactive, outreach monitoring protocol for new advanced therapy starts in IBD to assess clinical response and facilitate responsive disease management.
Methods: IBD patients complete 24 weeks of outreach monitoring after starting a new advanced therapy. PRO’s are collected on days 0, 3, and 7, and every 2 weeks thereafter. Serum labs are collected at baseline and weeks 4, 8, 12, 16, and 24 and FCP is collected at baseline and weeks 8, 16, and 24. Self-reported medication adherence is assessed at baseline and weeks 12 and 24. Endoscopy is booked 6-12 months from the start date. Granular clinical reports summarizing these results and the dates of last IBD review and last flare are sent to each treating gastroenterologist via our electronic medical record.
Results: 62 patients have been monitored with our protocol on the following biologic and small molecule therapies: ustekinumab (n = 30), tofacitinib (n = 14), risankizumab (n = 11), upacitinib (n = 4), vedolizumab (n = 2), and infliximab (n = 1). 41 patients (66.1%) had failed 1+ prior advanced therapies and 7 (11.3%) had prior IBD surgery. In 5 cases (8.1%), the patient was switched from their initial agent before 24 weeks due to lack of response. 54 patients (87.0%) complied with all FCP collections and 60 (96.8%) completed 95+% of PRO’s. Per STRIDE-II definitions, 38 patients (61.3%) demonstrated clinical response during the protocol and 20 (35.1%) achieved and maintained remission at 24 weeks. 14 patients (22.6%) received steroid courses, 9 (14.5%) presented to an emergency department, and 5 (8.1%) were hospitalized for their IBD during monitoring.
Discussion: Our STRIDE II based outreach monitoring protocol provided granular detail of clinical response to advanced IBD therapies to treating physicians. Patients were highly compliant with serial collection of PRO’s, FCP, and serum labs. This protocol allows for highly responsive disease management for IBD patients and may lead to treatment-specific monitoring recommendations and reduced healthcare utilization.

Figure: Figure 1. An overview of our proactive outreach monitoring protocol for IBD patients starting on new advanced therapies.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Scott MacKay indicated no relevant financial relationships.
Denise Parsons: Pfizer – Provides funding for salary..
Frank Hoentjen: Abbvie – Advisory Committee/Board Member, Grant/Research Support. Amgen – Advisory Committee/Board Member. Celltrion – Advisory Committee/Board Member. Janssen – Advisory Committee/Board Member, Grant/Research Support. Pendopharm – Advisory Committee/Board Member. Pfizer – Advisory Committee/Board Member, Grant/Research Support. Takeda – Advisory Committee/Board Member.
Levinus Dieleman: Abbvie – Advisory Committee/Board Member. Janssen – Advisory Committee/Board Member.
Michal Gozdzik: Pfizer – Grant/Research Support.
Karen Kroeker: Abbvie – Advisory Committee/Board Member. Celltrion – Advisory Committee/Board Member. Janssen – Advisory Committee/Board Member. Pfizer – Speakers Bureau. Takeda – Advisory Committee/Board Member.
Karen Wong: Abbvie – Speakers Bureau. Janssen – Advisory Committee/Board Member, Speakers Bureau.
Todd McMullen: Access Point of Care Services Inc. – Owner/Ownership Interest. Eli Lilly – Consultant. Pavonis Diagnostics Inc. – Owner/Ownership Interest.
Farhad Peerani: Abbvie – Advisory Committee/Board Member. BioJAMP – Advisory Committee/Board Member. Janssen – Advisory Committee/Board Member. Pfizer – Advisory Committee/Board Member. Takeda – Advisory Committee/Board Member.
Brendan Halloran: Abbvie – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Celltrion – Advisory Committee/Board Member, Speakers Bureau. Ferring – Advisory Committee/Board Member, Speakers Bureau. Fujifilm – Speakers Bureau. Innomar – Advisory Committee/Board Member. Janssen – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Pendopharm – Speakers Bureau. Pfizer – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Shire – Speakers Bureau. Takeda – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau.
Scott MacKay, MD, Denise Parsons, BA, Frank Hoentjen, MD, PhD, Levinus Dieleman, MD, PhD, Michal Gozdzik, MD, Karen Kroeker, MD, MSc, Karen Wong, MD, Todd McMullen, MD, Farhad Peerani, MD, Brendan Halloran, MD. P2635 - A New, Multifaceted STRIDE II-Based Monitoring Protocol for Advanced Therapy Starts in Inflammatory Bowel Disease, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
