Monday Poster Session
Category: IBD
P2647 - Risk of Herpes Zoster Among Adults With Inflammatory Bowel Disease After Initiating Immunosuppressive Therapy
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

Has Audio
- NS
Nikita Stempniewicz, MSc
GSK
Philadelphia, PA
Presenting Author(s)
Justin Gatwood, PhD, MPH1, Yong Zhu, PhD2, Andrea Steffens, MPH2, Stephanie Gallagher, MPH2, Mary DuCharme, MS2, Nikita Stempniewicz, MSc1
1GSK, Philadelphia, PA; 2Optum Life Sciences, Eden Prairie, MN
Introduction: Compared to the immunocompetent US population aged ≥50 years, herpes zoster (HZ) risk is higher among adults with inflammatory bowel disease (IBD). However, while estimates from previous studies provided guidance on HZ risk, the association of this risk following the initiation of immunosuppressive (IS) medications has yet to be fully explored.
Methods: This was a retrospective cohort study of commercial and Medicare Advantage with Part D health plan members using October 2015-December 2022 administrative claims data. Adults ≥18 years old, with ≥1 claim for an IS medication with a diagnosis for Crohn’s disease or ulcerative colitis (UC), and continuous enrollment for ≥12 months prior to the first IS medication fill (index date) were eligible. Patients were followed until the earlier of an HZ diagnosis or vaccination, pregnancy, end of enrollment or study period, or death. HZ incidence rates (IR) per 1,000 person-years were calculated and multivariable Cox proportional hazard models produced adjusted hazard ratios (aHRs) that controlled for baseline characteristics and time-varying IS status during follow-up.
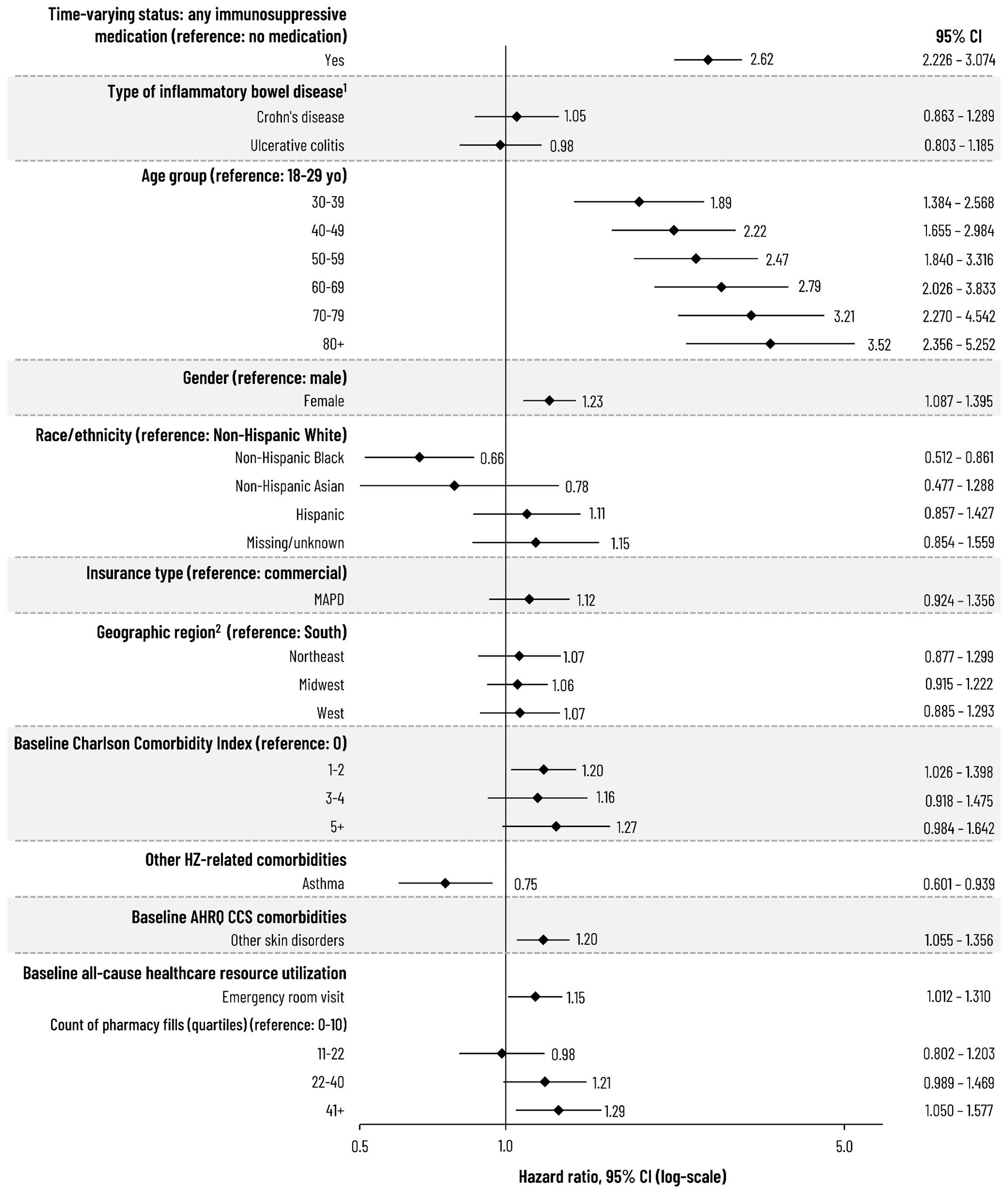
Results: A total of 28,852 patients were analyzed and followed for a median of 590 days (interquartile range [IQR]: 240-1,186 days) during which 1,050 HZ events were observed, the median time to which was 494 days (IQR: 205-957 days) from IS medication initiation. The overall unadjusted HZ IR was 17.3 (95% confidence interval [CI]: 16.23-18.33) and IRs tended to increase with age and the number of IS medication categories but varied by index medication category. By condition, the unadjusted IRs were 17.6 (95% CI: 16.26-19.00) and 17.0 (95% CI: 15.57-18.49) for Crohn’s disease and UC, respectively. Across all patients, the aHR was 2.62 (95% CI: 2.226-3.074) based on time-varying use of any (vs no) IS medication (Figure), and aHRs tended to increase with age and were also higher among females vs males but lower among non-Hispanic Black adults vs non-Hispanic White adults. Following the HZ event, 10.3% had HZ ophthalmicus (among those with ≥30 days of follow-up post-HZ diagnosis) and, among those with ≥6 months of follow-up after the HZ event, 19.3% developed post-herpetic neuralgia.
Discussion: Adults with IBD have a high risk of HZ following IS medication initiation, reinforcing the need for HZ prevention when IS therapy is being considered, irrespective of patient age.
Funding: GSK (GSK study identifier: VEO-000613)
Disclosures:
Justin Gatwood, PhD, MPH1, Yong Zhu, PhD2, Andrea Steffens, MPH2, Stephanie Gallagher, MPH2, Mary DuCharme, MS2, Nikita Stempniewicz, MSc1. P2647 - Risk of Herpes Zoster Among Adults With Inflammatory Bowel Disease After Initiating Immunosuppressive Therapy, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1GSK, Philadelphia, PA; 2Optum Life Sciences, Eden Prairie, MN
Introduction: Compared to the immunocompetent US population aged ≥50 years, herpes zoster (HZ) risk is higher among adults with inflammatory bowel disease (IBD). However, while estimates from previous studies provided guidance on HZ risk, the association of this risk following the initiation of immunosuppressive (IS) medications has yet to be fully explored.
Methods: This was a retrospective cohort study of commercial and Medicare Advantage with Part D health plan members using October 2015-December 2022 administrative claims data. Adults ≥18 years old, with ≥1 claim for an IS medication with a diagnosis for Crohn’s disease or ulcerative colitis (UC), and continuous enrollment for ≥12 months prior to the first IS medication fill (index date) were eligible. Patients were followed until the earlier of an HZ diagnosis or vaccination, pregnancy, end of enrollment or study period, or death. HZ incidence rates (IR) per 1,000 person-years were calculated and multivariable Cox proportional hazard models produced adjusted hazard ratios (aHRs) that controlled for baseline characteristics and time-varying IS status during follow-up.
Results: A total of 28,852 patients were analyzed and followed for a median of 590 days (interquartile range [IQR]: 240-1,186 days) during which 1,050 HZ events were observed, the median time to which was 494 days (IQR: 205-957 days) from IS medication initiation. The overall unadjusted HZ IR was 17.3 (95% confidence interval [CI]: 16.23-18.33) and IRs tended to increase with age and the number of IS medication categories but varied by index medication category. By condition, the unadjusted IRs were 17.6 (95% CI: 16.26-19.00) and 17.0 (95% CI: 15.57-18.49) for Crohn’s disease and UC, respectively. Across all patients, the aHR was 2.62 (95% CI: 2.226-3.074) based on time-varying use of any (vs no) IS medication (Figure), and aHRs tended to increase with age and were also higher among females vs males but lower among non-Hispanic Black adults vs non-Hispanic White adults. Following the HZ event, 10.3% had HZ ophthalmicus (among those with ≥30 days of follow-up post-HZ diagnosis) and, among those with ≥6 months of follow-up after the HZ event, 19.3% developed post-herpetic neuralgia.
Discussion: Adults with IBD have a high risk of HZ following IS medication initiation, reinforcing the need for HZ prevention when IS therapy is being considered, irrespective of patient age.
Funding: GSK (GSK study identifier: VEO-000613)

Figure: Figure. Risk of HZ among Adults with Inflammatory Bowel Disease
1. Comparator for type of inflammatory bowel disease was the presence of the individual condition versus those without that diagnosis.
2. South region includes patients from "other" region.
AHRQ, Agency for Healthcare Research and Quality; CI, confidence interval; CCS, Clinical Classifications Software; HZ, herpes zoster; MAPD, Medicare Advantage Part D; yo, years of age.
1. Comparator for type of inflammatory bowel disease was the presence of the individual condition versus those without that diagnosis.
2. South region includes patients from "other" region.
AHRQ, Agency for Healthcare Research and Quality; CI, confidence interval; CCS, Clinical Classifications Software; HZ, herpes zoster; MAPD, Medicare Advantage Part D; yo, years of age.
Disclosures:
Justin Gatwood: AstraZeneca – Grant/Research Support. Genentech – Consultant. GSK – Employee, Stock Options. Janssen – Advisory Committee/Board Member. Merck & Co. – Advisory Committee/Board Member, Grant/Research Support.
Yong Zhu: GSK – Optum received funding from GSK to conduct the study. Optum – Employee, Stock Options.
Andrea Steffens: GSK – Optum received funding from GSK to conduct the study. Optum – Employee, Stock Options.
Stephanie Gallagher: GSK – Optum received funding from GSK to conduct the study. Optum – Employee, Stock Options.
Mary DuCharme: GSK – Optum received funding from GSK to conduct the study. Optum – Employee, Stock Options.
Nikita Stempniewicz: GSK – Employee, Stock Options.
Justin Gatwood, PhD, MPH1, Yong Zhu, PhD2, Andrea Steffens, MPH2, Stephanie Gallagher, MPH2, Mary DuCharme, MS2, Nikita Stempniewicz, MSc1. P2647 - Risk of Herpes Zoster Among Adults With Inflammatory Bowel Disease After Initiating Immunosuppressive Therapy, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
