Monday Poster Session
Category: IBD
P2591 - Clinical Trial Characteristics of Inflammatory Bowel Disease Trials Influencing Reporting and Representation of Race and Ethnicity on ClinicalTrials.gov
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio
- KF
Kush Fansiwala, MD
University of California Los Angeles David Geffen School of Medicine
Los Angeles, CA
Presenting Author(s)
Kush Fansiwala, MD, Prince Shah-Riar, MD, Jenny Sauk, MD, Berkeley Limketkai, MD, PhD
University of California Los Angeles David Geffen School of Medicine, Los Angeles, CA
Introduction: Research has highlighted a lack of reporting and recruitment of minority populations in inflammatory bowel disease (IBD) studies. Given this, we identified trial characteristics contributing to minority reporting and representation on ClinicalTrials.gov.
Methods: We identified early phase 1-phase 4, adult-only trials from 01/01/2010 to present with results published on ClinicalTrials.gov. Search terms included “IBD”, “ulcerative colitis”, and “Crohn’s disease”. Studies with less than 10 participants were excluded. Study start date after NIH race/ethnicity reporting mandate of 1/18/2017, sponsor, multi-site recruitment, study location, disease, delivery of intervention, and race/ethnicity reporting and breakdown were collected. Outcomes of interest were study characteristics influencing reporting of race/ethnicity and adequate representation (defined by meeting established racial/ethnic prevalence of IBD in the United States) on ClinicalTrials.gov. Chi-squared test analyzed categorical variables and multivariable logistic regression was conducted for significant variables on univariate analysis.
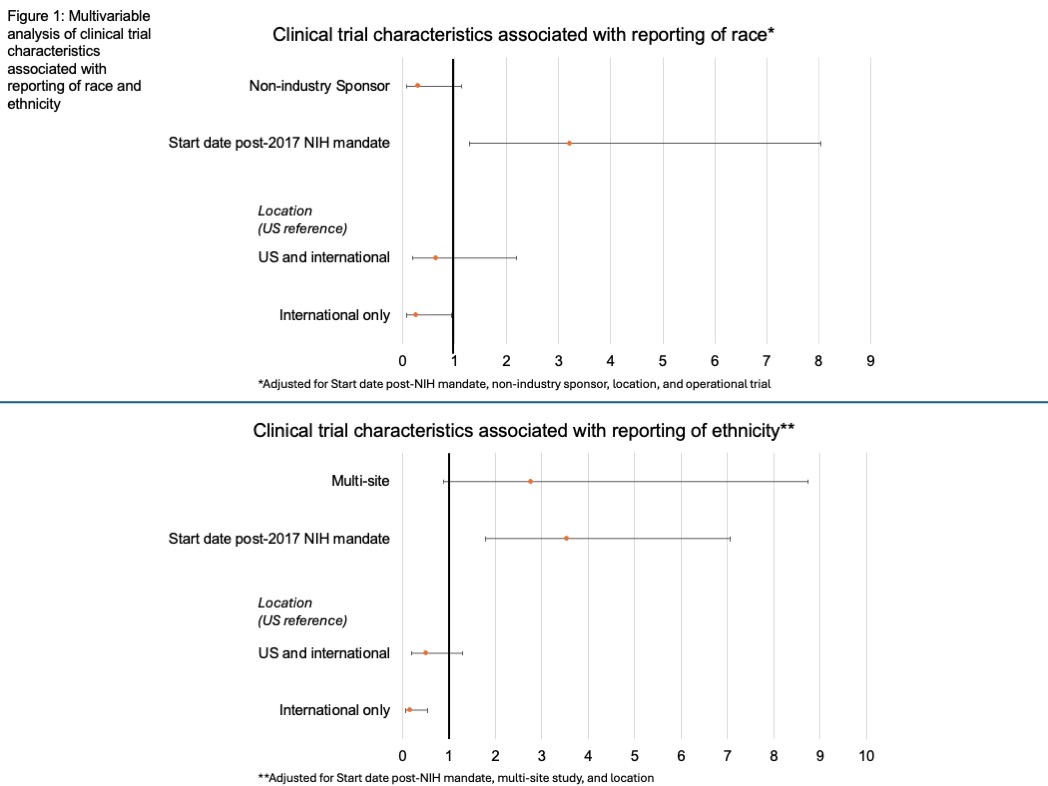
Results: Of 205 total trials identified, 75% reported race and 44% reported ethnicity. Multivariable analysis (Figure 1) showed post-NIH mandate start date increased reporting of race (OR 3.22; CI 1.29-8.04) while international study locations were associated with decreased reporting (OR 0.27; CI 0.08-0.94). For ethnicity reporting, start date post-NIH mandate increased odds (OR 3.55; CI 1.79-7.05) and study location (OR 0.17, CI 0.06-0.55) decreased odds of reporting. Among US only studies reporting race,14, 62, and 35% had adequate representation of Black, AAPI, and American Indian/Native American populations, respectively. 57% of US only studies reporting ethnicity had adequate Hispanic representation. No characteristics influenced adequate representation of race in all studies (Table 1), though international only studies were associated with lower adequate Hispanic representation (p=0.03).
Discussion: Our findings reveal increased race/ethnicity reporting on ClinicalTrials.gov after the 2017 NIH reporting mandate. Despite the mandate, no clinical trial characteristics affected representation, apart from international study location, likely due to limited Hispanic populations in these areas. Among US studies, adequate representation, especially of Black participants, remains low. This underscores the need for investment into recruitment strategies for minority representation in IBD trials.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Kush Fansiwala, MD, Prince Shah-Riar, MD, Jenny Sauk, MD, Berkeley Limketkai, MD, PhD. P2591 - Clinical Trial Characteristics of Inflammatory Bowel Disease Trials Influencing Reporting and Representation of Race and Ethnicity on ClinicalTrials.gov, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
University of California Los Angeles David Geffen School of Medicine, Los Angeles, CA
Introduction: Research has highlighted a lack of reporting and recruitment of minority populations in inflammatory bowel disease (IBD) studies. Given this, we identified trial characteristics contributing to minority reporting and representation on ClinicalTrials.gov.
Methods: We identified early phase 1-phase 4, adult-only trials from 01/01/2010 to present with results published on ClinicalTrials.gov. Search terms included “IBD”, “ulcerative colitis”, and “Crohn’s disease”. Studies with less than 10 participants were excluded. Study start date after NIH race/ethnicity reporting mandate of 1/18/2017, sponsor, multi-site recruitment, study location, disease, delivery of intervention, and race/ethnicity reporting and breakdown were collected. Outcomes of interest were study characteristics influencing reporting of race/ethnicity and adequate representation (defined by meeting established racial/ethnic prevalence of IBD in the United States) on ClinicalTrials.gov. Chi-squared test analyzed categorical variables and multivariable logistic regression was conducted for significant variables on univariate analysis.
Results: Of 205 total trials identified, 75% reported race and 44% reported ethnicity. Multivariable analysis (Figure 1) showed post-NIH mandate start date increased reporting of race (OR 3.22; CI 1.29-8.04) while international study locations were associated with decreased reporting (OR 0.27; CI 0.08-0.94). For ethnicity reporting, start date post-NIH mandate increased odds (OR 3.55; CI 1.79-7.05) and study location (OR 0.17, CI 0.06-0.55) decreased odds of reporting. Among US only studies reporting race,14, 62, and 35% had adequate representation of Black, AAPI, and American Indian/Native American populations, respectively. 57% of US only studies reporting ethnicity had adequate Hispanic representation. No characteristics influenced adequate representation of race in all studies (Table 1), though international only studies were associated with lower adequate Hispanic representation (p=0.03).
Discussion: Our findings reveal increased race/ethnicity reporting on ClinicalTrials.gov after the 2017 NIH reporting mandate. Despite the mandate, no clinical trial characteristics affected representation, apart from international study location, likely due to limited Hispanic populations in these areas. Among US studies, adequate representation, especially of Black participants, remains low. This underscores the need for investment into recruitment strategies for minority representation in IBD trials.

Figure: Figure 1: Multivariable analysis of clinical trial characteristics associated with reporting of race and ethnicity
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Kush Fansiwala indicated no relevant financial relationships.
Prince Shah-Riar indicated no relevant financial relationships.
Jenny Sauk indicated no relevant financial relationships.
Berkeley Limketkai indicated no relevant financial relationships.
Kush Fansiwala, MD, Prince Shah-Riar, MD, Jenny Sauk, MD, Berkeley Limketkai, MD, PhD. P2591 - Clinical Trial Characteristics of Inflammatory Bowel Disease Trials Influencing Reporting and Representation of Race and Ethnicity on ClinicalTrials.gov, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
