Monday Poster Session
Category: General Endoscopy
P2389 - The Impact of GLP-1 Receptor Agonists on Retained Gastric Contents, Aborted Procedures, and Aspiration During Esophagogastroduodenoscopy: A Systematic Review and Meta-Analysis
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

Has Audio

Eleazar Enrique Montalvan-Sanchez, MD
Yale Digestive Diseases
New Haven, CT
Presenting Author(s)
Eleazar Enrique. Montalvan-Sanchez, MD1, Dalton A.. Norwood, MD2, Renato Beas, MD3, Malcolm B. Chapman, MBA, MD4, Daniela M. Montalvan-Sanchez, MD5, Leandro Sierra, MD6, Mirian Ramirez, 7, Sergio A. Sánchez-Luna, MD8, Shajan Peter, MD8, Douglas Morgan, MD8, Ramzi Mulki, MD8, Michael Dougherty, MD9
1Yale Digestive Diseases, New Haven, CT; 2UAB Minority Health & Health Equity Research Center, The University of Alabama at Birmingham Heersink School of Medicine, Birmingham, AL; 3Washington University School of Medicine in St. Louis / Barnes-Jewish Hospital, St. Louis, MO; 4University of Alabama at Birmingham Heersink School of Medicine, Birmingham, AL; 5National Autonomous University of Honduras, Arlington, TX; 6Cleveland Clinic Foundation, Cleveland, OH; 7Ruth Lilly Medical Library, Indiana University School of Medicine, Indianapolis, IN; 8Basil I. Hirschowitz Endoscopic Center of Excellence, The University of Alabama at Birmingham Heersink School of Medicine, Birmingham, AL; 9University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, NC
Introduction: Glucagon-like peptide-1 receptor agonists (GLP-1RAs) are widely used for treating type 2 diabetes mellitus (T2DM) and obesity, and are being explored for other conditions. Their effect on delaying gastric emptying raises concerns regarding retained gastric contents (RGC), perioperative pulmonary aspiration (PA), particularly during esophagogastroduodenoscopy (EGD). We aimed to systematically review and conduct a meta-analysis of the association between GLP-1RA use and the incidence of gastric retained contents (RGC), incomplete EGD, repeat EGD, and procedure-related adverse events (AEs).
Methods: We searched PubMed, MEDLINE via Ovid, Embase via Elsevier, Web of Science, Scopus and Cochrane library from their inception to May 23, 2024. Trial registries were searched until the same date. We included studies of participants aged 18 years or older who underwent EGD while taking GLP-1RAs, if incidence of the outcomes could be calculated. At least two reviewers independently screened and selected the studies, extracted data on study design, patient characteristics, and outcomes, and assessed the risk of bias using the Newcastle-Ottawa Scale.
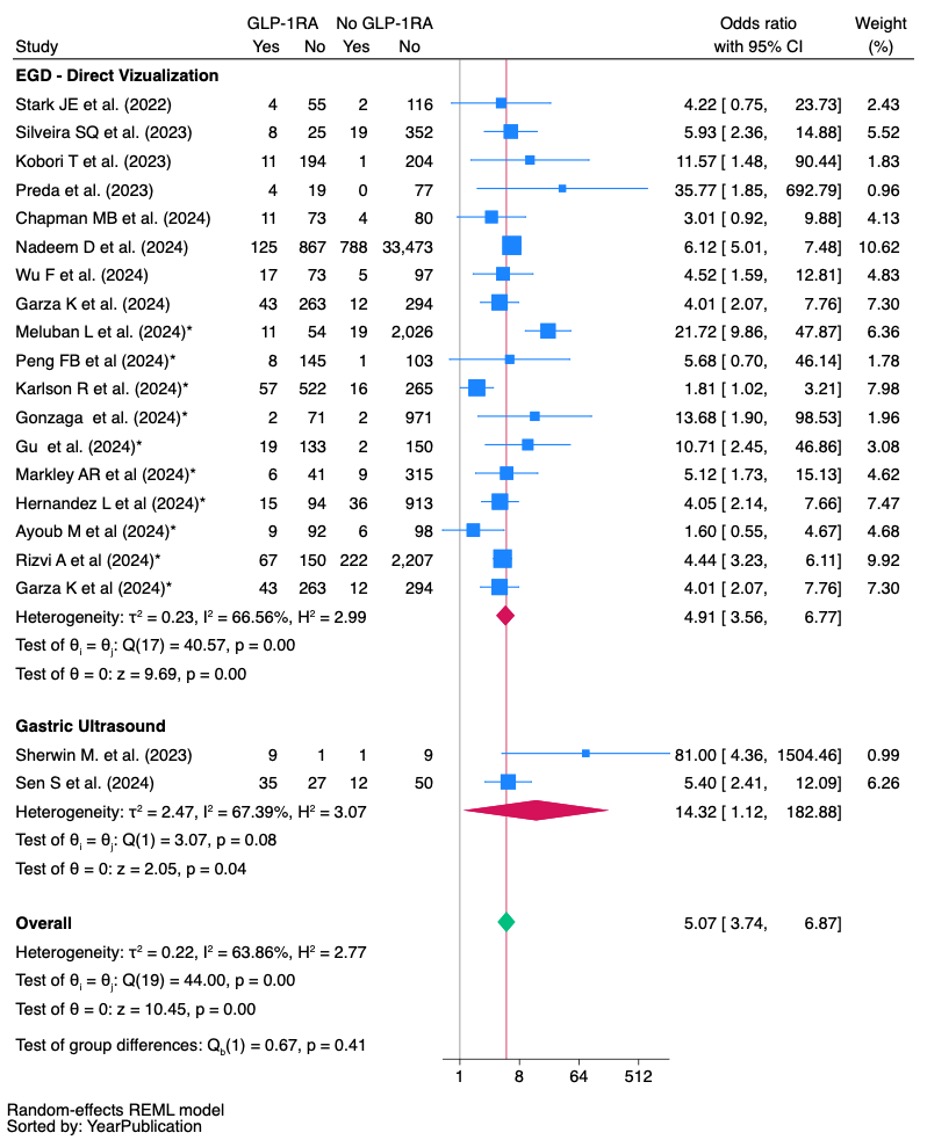
Results: We included 29 studies (n=137,614 patients; 44,101 GLP-1RA users). 24 studies evaluated the use of any GLP-1RA while 5 focused on once-weekly GLP-1RA. GLP-1RA use was associated with a higher prevalence of RGC (14.08% vs. 2.99%; OR, 5.07,95%CI:3.74-6.87), particularly solid contents (21.58% vs. 2.55%, OR: 4.89,95%CI:2.67-8.69). GLP-1RAs increased the risk of aborted procedures (1.42% vs. 0.17%; OR, 4.76,95%CI:2.76-8.14). The aspiration frequency was numerically higher in the GLP-1RA group (127/38,365 cases) compared to the non-GLP1-RA group (96/51,129 cases), but not statistically significant (OR: 1.53, 95%CI:0.92-2.56).
Discussion: Our study highlighted three key dimensions related to the clinical implications of delayed gastric emptying from GLP-1 receptor agonist (GLP-1 RA) use. Firstly, we noted a significantly higher incidence and risk of retained gastric contents (RGC) in patients treated with GLP-1 RA agents. Despite this increase, the elevated RGC did not correspond to a rise in aspiration-related adverse effects. However, the increased incidence of RGC was clinically significant as it led to more aborted procedures. Additionally, RGC compromised the effectiveness of the procedures by obscuring the gastric mucosa, which is essential for accurately diagnosing small lesions.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Eleazar Enrique. Montalvan-Sanchez, MD1, Dalton A.. Norwood, MD2, Renato Beas, MD3, Malcolm B. Chapman, MBA, MD4, Daniela M. Montalvan-Sanchez, MD5, Leandro Sierra, MD6, Mirian Ramirez, 7, Sergio A. Sánchez-Luna, MD8, Shajan Peter, MD8, Douglas Morgan, MD8, Ramzi Mulki, MD8, Michael Dougherty, MD9. P2389 - The Impact of GLP-1 Receptor Agonists on Retained Gastric Contents, Aborted Procedures, and Aspiration During Esophagogastroduodenoscopy: A Systematic Review and Meta-Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Yale Digestive Diseases, New Haven, CT; 2UAB Minority Health & Health Equity Research Center, The University of Alabama at Birmingham Heersink School of Medicine, Birmingham, AL; 3Washington University School of Medicine in St. Louis / Barnes-Jewish Hospital, St. Louis, MO; 4University of Alabama at Birmingham Heersink School of Medicine, Birmingham, AL; 5National Autonomous University of Honduras, Arlington, TX; 6Cleveland Clinic Foundation, Cleveland, OH; 7Ruth Lilly Medical Library, Indiana University School of Medicine, Indianapolis, IN; 8Basil I. Hirschowitz Endoscopic Center of Excellence, The University of Alabama at Birmingham Heersink School of Medicine, Birmingham, AL; 9University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, NC
Introduction: Glucagon-like peptide-1 receptor agonists (GLP-1RAs) are widely used for treating type 2 diabetes mellitus (T2DM) and obesity, and are being explored for other conditions. Their effect on delaying gastric emptying raises concerns regarding retained gastric contents (RGC), perioperative pulmonary aspiration (PA), particularly during esophagogastroduodenoscopy (EGD). We aimed to systematically review and conduct a meta-analysis of the association between GLP-1RA use and the incidence of gastric retained contents (RGC), incomplete EGD, repeat EGD, and procedure-related adverse events (AEs).
Methods: We searched PubMed, MEDLINE via Ovid, Embase via Elsevier, Web of Science, Scopus and Cochrane library from their inception to May 23, 2024. Trial registries were searched until the same date. We included studies of participants aged 18 years or older who underwent EGD while taking GLP-1RAs, if incidence of the outcomes could be calculated. At least two reviewers independently screened and selected the studies, extracted data on study design, patient characteristics, and outcomes, and assessed the risk of bias using the Newcastle-Ottawa Scale.
Results: We included 29 studies (n=137,614 patients; 44,101 GLP-1RA users). 24 studies evaluated the use of any GLP-1RA while 5 focused on once-weekly GLP-1RA. GLP-1RA use was associated with a higher prevalence of RGC (14.08% vs. 2.99%; OR, 5.07,95%CI:3.74-6.87), particularly solid contents (21.58% vs. 2.55%, OR: 4.89,95%CI:2.67-8.69). GLP-1RAs increased the risk of aborted procedures (1.42% vs. 0.17%; OR, 4.76,95%CI:2.76-8.14). The aspiration frequency was numerically higher in the GLP-1RA group (127/38,365 cases) compared to the non-GLP1-RA group (96/51,129 cases), but not statistically significant (OR: 1.53, 95%CI:0.92-2.56).
Discussion: Our study highlighted three key dimensions related to the clinical implications of delayed gastric emptying from GLP-1 receptor agonist (GLP-1 RA) use. Firstly, we noted a significantly higher incidence and risk of retained gastric contents (RGC) in patients treated with GLP-1 RA agents. Despite this increase, the elevated RGC did not correspond to a rise in aspiration-related adverse effects. However, the increased incidence of RGC was clinically significant as it led to more aborted procedures. Additionally, RGC compromised the effectiveness of the procedures by obscuring the gastric mucosa, which is essential for accurately diagnosing small lesions.

Figure: The odds ratio (OR) for the occurrence of RGC in the GLP-1RA group compared to the control group by visualization methods. Random effects REML Model, Sorted by year of publication.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Eleazar Montalvan-Sanchez indicated no relevant financial relationships.
Dalton Norwood indicated no relevant financial relationships.
Renato Beas indicated no relevant financial relationships.
Malcolm Chapman indicated no relevant financial relationships.
Daniela Montalvan-Sanchez indicated no relevant financial relationships.
Leandro Sierra indicated no relevant financial relationships.
Mirian Ramirez indicated no relevant financial relationships.
Sergio A. Sánchez-Luna indicated no relevant financial relationships.
Shajan Peter: Olympus – Consultant.
Douglas Morgan: American Molecular Laboratory – Investigator-initiated study, company donated analysis. Freenome, Inc – Site PI, industry CRC diagnostics trial. Panbela Therapeutics – NCI-funded study, company donated study drug to the University. Thorne Research, Inc – NCI-funded study, company donated study drug to the University.
Ramzi Mulki indicated no relevant financial relationships.
Michael Dougherty indicated no relevant financial relationships.
Eleazar Enrique. Montalvan-Sanchez, MD1, Dalton A.. Norwood, MD2, Renato Beas, MD3, Malcolm B. Chapman, MBA, MD4, Daniela M. Montalvan-Sanchez, MD5, Leandro Sierra, MD6, Mirian Ramirez, 7, Sergio A. Sánchez-Luna, MD8, Shajan Peter, MD8, Douglas Morgan, MD8, Ramzi Mulki, MD8, Michael Dougherty, MD9. P2389 - The Impact of GLP-1 Receptor Agonists on Retained Gastric Contents, Aborted Procedures, and Aspiration During Esophagogastroduodenoscopy: A Systematic Review and Meta-Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
