Monday Poster Session
Category: Esophagus
P2234 - The Incidence of Adverse Events Associated With Three-Stage Esophageal Balloon Dilators: A Retrospective Review of the MAUDE Database
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

Has Audio

Riya M. Bhat, BA
University of Missouri - Kansas City School of Medicine
Kansas City, MO
Presenting Author(s)
Islam Mohamed, MD1, Samuel J. Kim, BA2, Riya M. Bhat, BA3, Husam Abu Suilik, 4, Hazem Abosheiashaa, MD, MSc5, Ahmed E. Salem, MBBCh6, Fouad Jaber, MD3, Ahmed El Telbany, MD, MPH7, Dushyant S. Dahiya, MD8, Hassan Ghoz, MD3
1University of Missouri, Columbia, MO; 2University of Missouri-Kansas City School of Medicine, Kansas City, MO; 3University of Missouri - Kansas City School of Medicine, Kansas City, MO; 4Hashemite University, Zaqra, 'Amman, Jordan; 5Icahn School of Medicine at Mount Sinai, New York, NY; 6Maimonides Medical Center, Brooklyn, NY; 7University of New Mexico, Albuquerque, NM; 8The University of Kansas School of Medicine, Kansas City, KS
Introduction: Esophageal balloon dilation involves the use of an inflatable balloon catheter to alleviate luminal narrowing in the esophagus. The dilator revolutionizes esophageal dilation as it can achieve three escalating diameters in one tool, reducing the need for multiple dilators. However, there are few studies discussing the complication rates related to these novel dilators. This study investigates device-related events and patient related complications associated with the three-stage esophageal balloon dilators during management of esophageal pathologies.
Methods: Data was queried from the Manufacturer and User Facility Device Experience (MAUDE) database from January 2017 to October 2023 for three-stage esophageal dilators. Incidents from 1556 patients were included in this study. Descriptive and regression analysis was conducted using SPSS software for patient and device-related adverse events.
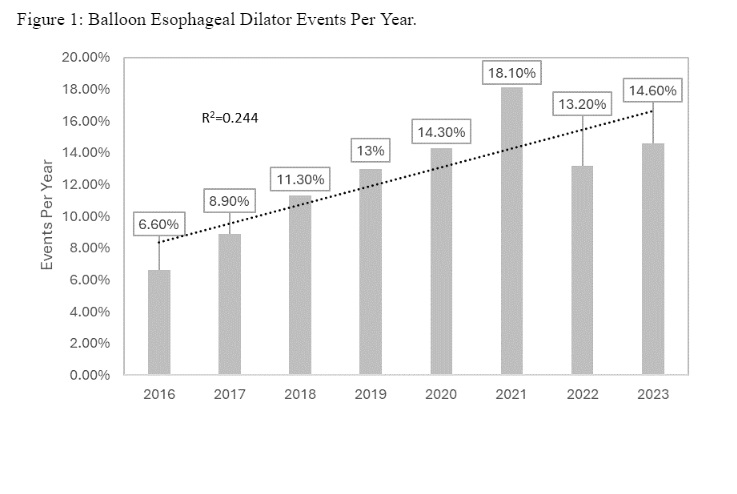
Results: The study identified prevalent device-related incidents and patient events associated with commonly used esophageal dilators in the US market. There were a total of 1931 events with the majority being device-related complications (n = 1865). Predominant device-related events included Burst Container or Vessel occurrences (27.5%), material punctures or holes (13.1%), fractures or cracks (11.2%), and fluid or blood leaks (10.99%). Patient-related events were rare (n = 66) with the predominant complication being foreign bodies found in the patient. Over the study period, there was a consistent increase in events by regression analysis (R2 = 0.244), with varying percentages recorded annually, culminating in 14.6% of events in 2023 and peaking at 18.10% in 2021.
Discussion: This analysis of adverse events associated with these dilators focuses on major aspects crucial for patient safety and device efficacy. Device-related complications, notably fractures/cracks and fluid/blood leaks, highlighted the urgency for design improvements to mitigate risks. Moreover, patient-related problems, particularly foreign body ingestion, underscore the need for stricter procedural protocols and increased clinical vigilance during dilation procedures. Three stage balloon dilators are a relatively new development in current medical technology, and a refined strategy aimed at enhancing device design while prioritizing patient safety is critical. Furthermore, comprehensive, prospective studies are required to identify possible problems and develop robust safety standards.
Disclosures:
Islam Mohamed, MD1, Samuel J. Kim, BA2, Riya M. Bhat, BA3, Husam Abu Suilik, 4, Hazem Abosheiashaa, MD, MSc5, Ahmed E. Salem, MBBCh6, Fouad Jaber, MD3, Ahmed El Telbany, MD, MPH7, Dushyant S. Dahiya, MD8, Hassan Ghoz, MD3. P2234 - The Incidence of Adverse Events Associated With Three-Stage Esophageal Balloon Dilators: A Retrospective Review of the MAUDE Database, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1University of Missouri, Columbia, MO; 2University of Missouri-Kansas City School of Medicine, Kansas City, MO; 3University of Missouri - Kansas City School of Medicine, Kansas City, MO; 4Hashemite University, Zaqra, 'Amman, Jordan; 5Icahn School of Medicine at Mount Sinai, New York, NY; 6Maimonides Medical Center, Brooklyn, NY; 7University of New Mexico, Albuquerque, NM; 8The University of Kansas School of Medicine, Kansas City, KS
Introduction: Esophageal balloon dilation involves the use of an inflatable balloon catheter to alleviate luminal narrowing in the esophagus. The dilator revolutionizes esophageal dilation as it can achieve three escalating diameters in one tool, reducing the need for multiple dilators. However, there are few studies discussing the complication rates related to these novel dilators. This study investigates device-related events and patient related complications associated with the three-stage esophageal balloon dilators during management of esophageal pathologies.
Methods: Data was queried from the Manufacturer and User Facility Device Experience (MAUDE) database from January 2017 to October 2023 for three-stage esophageal dilators. Incidents from 1556 patients were included in this study. Descriptive and regression analysis was conducted using SPSS software for patient and device-related adverse events.
Results: The study identified prevalent device-related incidents and patient events associated with commonly used esophageal dilators in the US market. There were a total of 1931 events with the majority being device-related complications (n = 1865). Predominant device-related events included Burst Container or Vessel occurrences (27.5%), material punctures or holes (13.1%), fractures or cracks (11.2%), and fluid or blood leaks (10.99%). Patient-related events were rare (n = 66) with the predominant complication being foreign bodies found in the patient. Over the study period, there was a consistent increase in events by regression analysis (R2 = 0.244), with varying percentages recorded annually, culminating in 14.6% of events in 2023 and peaking at 18.10% in 2021.
Discussion: This analysis of adverse events associated with these dilators focuses on major aspects crucial for patient safety and device efficacy. Device-related complications, notably fractures/cracks and fluid/blood leaks, highlighted the urgency for design improvements to mitigate risks. Moreover, patient-related problems, particularly foreign body ingestion, underscore the need for stricter procedural protocols and increased clinical vigilance during dilation procedures. Three stage balloon dilators are a relatively new development in current medical technology, and a refined strategy aimed at enhancing device design while prioritizing patient safety is critical. Furthermore, comprehensive, prospective studies are required to identify possible problems and develop robust safety standards.

Figure: The graph depicts the percentage of balloon esophageal dilator events per year.
Disclosures:
Islam Mohamed indicated no relevant financial relationships.
Samuel Kim indicated no relevant financial relationships.
Riya Bhat indicated no relevant financial relationships.
Husam Abu Suilik indicated no relevant financial relationships.
Hazem Abosheiashaa indicated no relevant financial relationships.
Ahmed Salem indicated no relevant financial relationships.
Fouad Jaber indicated no relevant financial relationships.
Ahmed El Telbany indicated no relevant financial relationships.
Dushyant Dahiya indicated no relevant financial relationships.
Hassan Ghoz indicated no relevant financial relationships.
Islam Mohamed, MD1, Samuel J. Kim, BA2, Riya M. Bhat, BA3, Husam Abu Suilik, 4, Hazem Abosheiashaa, MD, MSc5, Ahmed E. Salem, MBBCh6, Fouad Jaber, MD3, Ahmed El Telbany, MD, MPH7, Dushyant S. Dahiya, MD8, Hassan Ghoz, MD3. P2234 - The Incidence of Adverse Events Associated With Three-Stage Esophageal Balloon Dilators: A Retrospective Review of the MAUDE Database, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
