Monday Poster Session
Category: Colon
P1955 - A Multi-Epitope Self-Amplifying mRNA Vaccine for Colorectal Cancer Immunotherapy Designed by Computational Approaches
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

Has Audio
- ST
Salar Tofighi, MD
Saint Agnes Medical Center
Fresno, CA
Presenting Author(s)
Mohammad Ali Hosseini, MS1, Mahdi Malekpour, MD2, Amir Chitsaz, PharmD1, Yasamin Navabi, PharmD1, Mohammad Amin Zaree, PharmD1, Salar Tofighi, MD3, Saeed Soleymanjahi, MD4, Manica Negahdaripour, PhD, PharmD5
1Islamic Azad University, Tehran, Tehran, Iran; 2Shiraz University of Medical Sciences, Shiraz, Fars, Iran; 3Saint Agnes Medical Center, Fresno, CA; 4Yale New Haven Health, New Haven, CT; 5Saint Agnes Medical Center, Tehran, Tehran, Iran
Introduction: Colorectal cancer (CRC) ranks third in prevalence and fourth in mortality. Current therapies often yield inadequate responses. Self-amplifying mRNA vaccines may offer greater efficacy than non-replicating mRNAs, requiring lower doses and resulting in fewer side effects and reduced costs. This study designed a self-amplifying mRNA vaccine for CRC using immunoinformatic and bioinformatic methods.
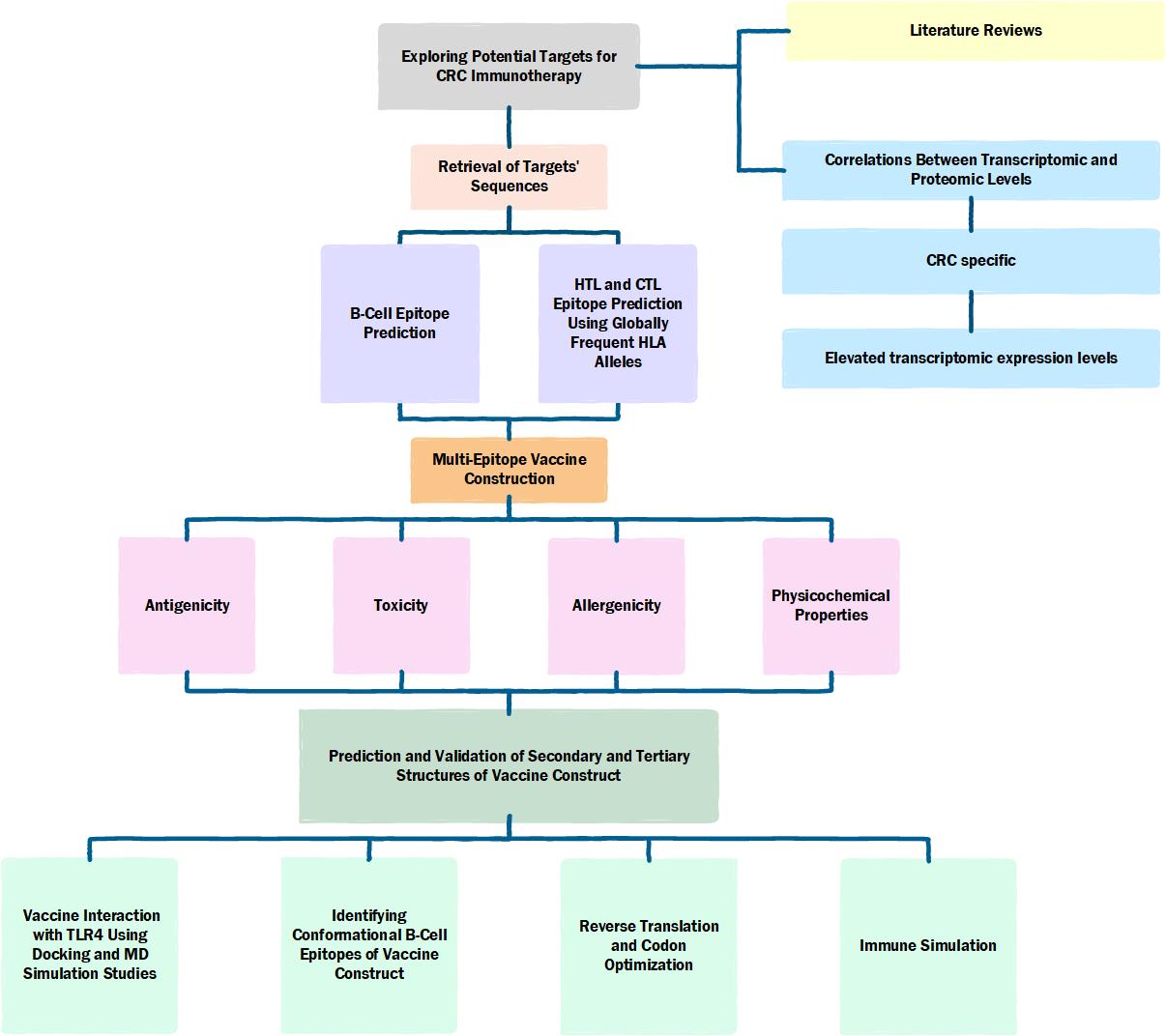
Methods: Candidate proteins were identified through literature review, transcriptomic expression levels, transcriptomic and proteomic correlation, CRC specificity, and antigenicity (Image). Sequences of extracellular portions were retrieved. Linear B cell, cytotoxic T lymphocyte (CTL), and helper T lymphocyte (HTL) epitopes were mapped. T cell epitopes interacting with globally prevalent HLA alleles were chosen to optimize vaccine coverage. Selected epitopes were linked, forming an mRNA vaccine comprising open reading frames, tissue plasminogen activator, MHC class I targeting domain, arranged epitopes, a self-amplifying replicase sequence, and TLR4 agonist (RS09) adjuvant. The construct was evaluated for antigenicity, toxicity, allergenicity, and physicochemical properties, with secondary and tertiary structures predicted. Vaccine interaction with TLR4 was analyzed via docking and MD simulations, and conformational B-cell epitopes were identified. Immune simulation and in silico cloning were conducted.
Results: Different epitopes were identified on ACE2, ANXA13, CTA1, and CTA2 proteins. Eight highly antigenic, non-toxic, and non-allergenic epitopes were selected. The chosen CTL and HTL epitopes were predicted to cover 92.38% and 74.13% of the global population, respectively. A candidate protein was engineered with appropriate immunological and physicochemical properties (Table), an accurate tertiary structure model, and strong binding affinity to TLR4. Simulations indicated that this vaccine could elicit a robust immune response.
Discussion: A self-amplifying mRNA vaccine was developed, showing potential efficiency. This study adds to the limited body of research on mRNA vaccine development. Further laboratory and clinical studies are required to validate these findings.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Mohammad Ali Hosseini, MS1, Mahdi Malekpour, MD2, Amir Chitsaz, PharmD1, Yasamin Navabi, PharmD1, Mohammad Amin Zaree, PharmD1, Salar Tofighi, MD3, Saeed Soleymanjahi, MD4, Manica Negahdaripour, PhD, PharmD5. P1955 - A Multi-Epitope Self-Amplifying mRNA Vaccine for Colorectal Cancer Immunotherapy Designed by Computational Approaches, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Islamic Azad University, Tehran, Tehran, Iran; 2Shiraz University of Medical Sciences, Shiraz, Fars, Iran; 3Saint Agnes Medical Center, Fresno, CA; 4Yale New Haven Health, New Haven, CT; 5Saint Agnes Medical Center, Tehran, Tehran, Iran
Introduction: Colorectal cancer (CRC) ranks third in prevalence and fourth in mortality. Current therapies often yield inadequate responses. Self-amplifying mRNA vaccines may offer greater efficacy than non-replicating mRNAs, requiring lower doses and resulting in fewer side effects and reduced costs. This study designed a self-amplifying mRNA vaccine for CRC using immunoinformatic and bioinformatic methods.
Methods: Candidate proteins were identified through literature review, transcriptomic expression levels, transcriptomic and proteomic correlation, CRC specificity, and antigenicity (Image). Sequences of extracellular portions were retrieved. Linear B cell, cytotoxic T lymphocyte (CTL), and helper T lymphocyte (HTL) epitopes were mapped. T cell epitopes interacting with globally prevalent HLA alleles were chosen to optimize vaccine coverage. Selected epitopes were linked, forming an mRNA vaccine comprising open reading frames, tissue plasminogen activator, MHC class I targeting domain, arranged epitopes, a self-amplifying replicase sequence, and TLR4 agonist (RS09) adjuvant. The construct was evaluated for antigenicity, toxicity, allergenicity, and physicochemical properties, with secondary and tertiary structures predicted. Vaccine interaction with TLR4 was analyzed via docking and MD simulations, and conformational B-cell epitopes were identified. Immune simulation and in silico cloning were conducted.
Results: Different epitopes were identified on ACE2, ANXA13, CTA1, and CTA2 proteins. Eight highly antigenic, non-toxic, and non-allergenic epitopes were selected. The chosen CTL and HTL epitopes were predicted to cover 92.38% and 74.13% of the global population, respectively. A candidate protein was engineered with appropriate immunological and physicochemical properties (Table), an accurate tertiary structure model, and strong binding affinity to TLR4. Simulations indicated that this vaccine could elicit a robust immune response.
Discussion: A self-amplifying mRNA vaccine was developed, showing potential efficiency. This study adds to the limited body of research on mRNA vaccine development. Further laboratory and clinical studies are required to validate these findings.

Figure: Flowchart for the designed study.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Mohammad Ali Hosseini indicated no relevant financial relationships.
Mahdi Malekpour indicated no relevant financial relationships.
Amir Chitsaz indicated no relevant financial relationships.
Yasamin Navabi indicated no relevant financial relationships.
Mohammad Amin Zaree indicated no relevant financial relationships.
Salar Tofighi indicated no relevant financial relationships.
Saeed Soleymanjahi indicated no relevant financial relationships.
Manica Negahdaripour indicated no relevant financial relationships.
Mohammad Ali Hosseini, MS1, Mahdi Malekpour, MD2, Amir Chitsaz, PharmD1, Yasamin Navabi, PharmD1, Mohammad Amin Zaree, PharmD1, Salar Tofighi, MD3, Saeed Soleymanjahi, MD4, Manica Negahdaripour, PhD, PharmD5. P1955 - A Multi-Epitope Self-Amplifying mRNA Vaccine for Colorectal Cancer Immunotherapy Designed by Computational Approaches, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
