Monday Poster Session
Category: Biliary/Pancreas
P1786 - Prescriber Beware: Rethinking the Routine Glucagon-Like Peptide-1 Receptor Agonists
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

Has Audio

Yousef Hreish, MD
University of Mississippi Medical Center
Jackson, MS
Presenting Author(s)
Yousef Hreish, MD1, Hamid Ullah, MBBS1, Faisal A.. Bukeirat, MD2
1University of Mississippi Medical Center, Jackson, MS; 2UMMC, Jackson, MS
Introduction: Glucagon-like peptide-1 receptor agonists (GLP-1RA) are currently the trending “miracle” drug everyone is seeking. GLP-1RA are being prescribed at unprecedented rates among various specialties as increasing data indicates health benefits. There are no current guidelines or practice requirements to determine which patients may be at risk of significant adverse events. Here we present a case of patient with newly diagnosed pancreatic adenocarcinoma who had been on an GLP-1RA for over one year prior to diagnosis.
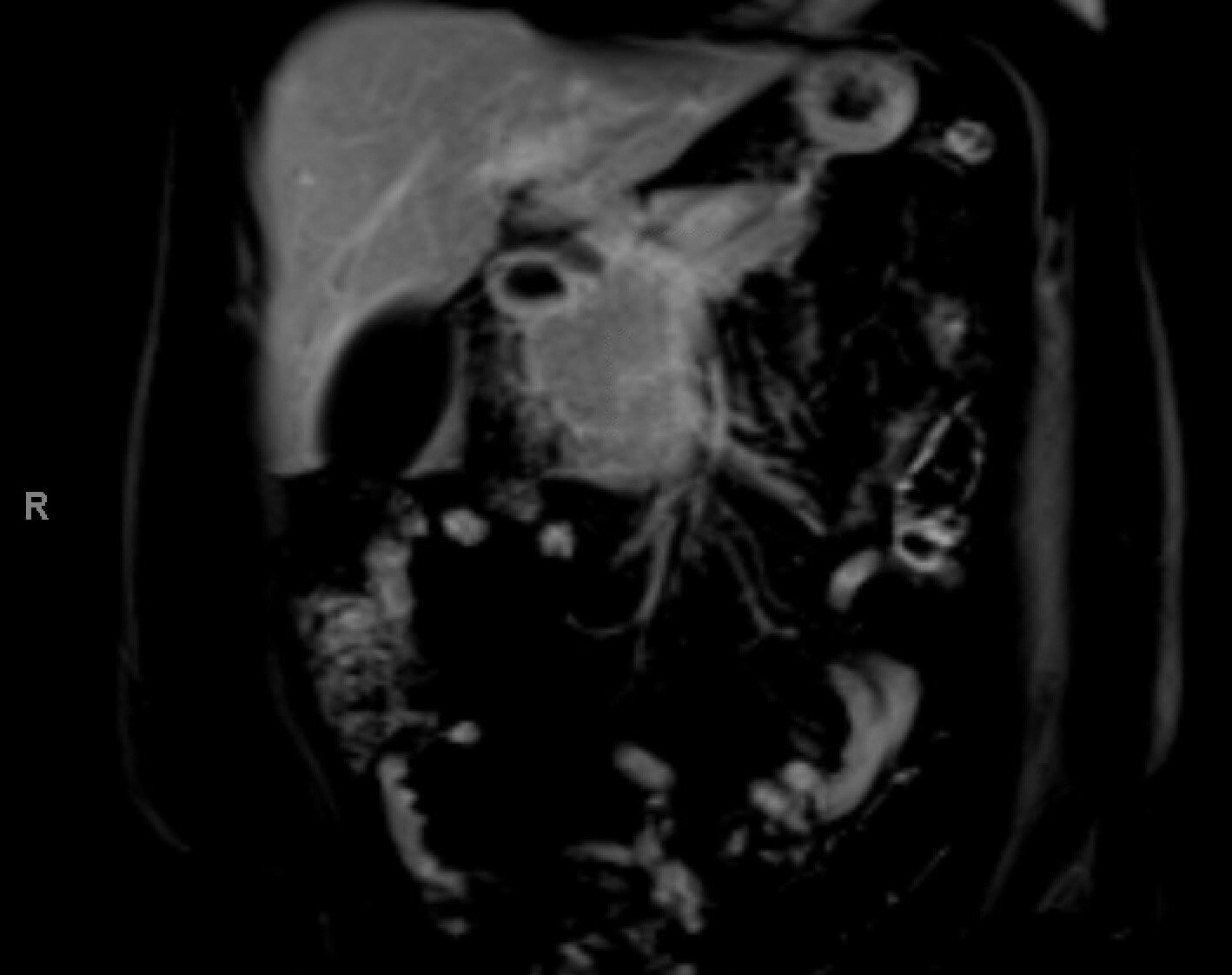
Case Description/Methods: 53 year old obese female with a history of breast cancer in remission, presents for ongoing 40 pound weight loss and painless jaundice despite being off of GLP-1RA for three months. Patient was on GLP-1RA for over 1 year for medically recommended weight loss that continued after stopping the medication. Imaging on presentation is notable for restrictive pancreatic head mass obstructing the common bile duct with 360 degree encasement of celiac bifurcation, common hepatic artery, and proximal splenic artery with extra hepatic ductal dilation and metastatic lesions through the liver. Lab findings most notable for bilirubin of 3. Patient underwent endoscopic ultrasound (EUS) with fine needle biopsy confirming pancreatic adenocarcinoma, which was peculiar in its inflammatory nature and in the fact it grew like a weed along the portal and biliary trees.
Discussion: The case raises concerns pertaining to the prescribing, administering and counseling of the use of GLP-1RA. In the realm of prescribing, there are no current practice requirements for these medications and little research to support an oncological association with GLP-1RA. This patient’s malignancy prior to the initiation of GLP-1RA poses the need to evaluate past medical histories and the synergistic impact that may be seen with GLP-1RA. Though a 16-year retrospective FDA Adverse Event Reporting System database shows no association of GLP-1RA and system organ classes,1 with an increasing number of patients being prescribed this medicine class and the case presented here, further studies are needed. Should pause be taken prior to initiation of GLP-1RA with any history of malignancies? Should additional imaging be completed prior to therapy initiation to assess intrabdominal anatomy and potential pathology? Though there are many benefits of GLP-1RAs this case poses these above questions that must be considered.
Disclosures:
Yousef Hreish, MD1, Hamid Ullah, MBBS1, Faisal A.. Bukeirat, MD2. P1786 - Prescriber Beware: Rethinking the Routine Glucagon-Like Peptide-1 Receptor Agonists, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1University of Mississippi Medical Center, Jackson, MS; 2UMMC, Jackson, MS
Introduction: Glucagon-like peptide-1 receptor agonists (GLP-1RA) are currently the trending “miracle” drug everyone is seeking. GLP-1RA are being prescribed at unprecedented rates among various specialties as increasing data indicates health benefits. There are no current guidelines or practice requirements to determine which patients may be at risk of significant adverse events. Here we present a case of patient with newly diagnosed pancreatic adenocarcinoma who had been on an GLP-1RA for over one year prior to diagnosis.
Case Description/Methods: 53 year old obese female with a history of breast cancer in remission, presents for ongoing 40 pound weight loss and painless jaundice despite being off of GLP-1RA for three months. Patient was on GLP-1RA for over 1 year for medically recommended weight loss that continued after stopping the medication. Imaging on presentation is notable for restrictive pancreatic head mass obstructing the common bile duct with 360 degree encasement of celiac bifurcation, common hepatic artery, and proximal splenic artery with extra hepatic ductal dilation and metastatic lesions through the liver. Lab findings most notable for bilirubin of 3. Patient underwent endoscopic ultrasound (EUS) with fine needle biopsy confirming pancreatic adenocarcinoma, which was peculiar in its inflammatory nature and in the fact it grew like a weed along the portal and biliary trees.
Discussion: The case raises concerns pertaining to the prescribing, administering and counseling of the use of GLP-1RA. In the realm of prescribing, there are no current practice requirements for these medications and little research to support an oncological association with GLP-1RA. This patient’s malignancy prior to the initiation of GLP-1RA poses the need to evaluate past medical histories and the synergistic impact that may be seen with GLP-1RA. Though a 16-year retrospective FDA Adverse Event Reporting System database shows no association of GLP-1RA and system organ classes,1 with an increasing number of patients being prescribed this medicine class and the case presented here, further studies are needed. Should pause be taken prior to initiation of GLP-1RA with any history of malignancies? Should additional imaging be completed prior to therapy initiation to assess intrabdominal anatomy and potential pathology? Though there are many benefits of GLP-1RAs this case poses these above questions that must be considered.

Figure: MRI Abdomen
Disclosures:
Yousef Hreish indicated no relevant financial relationships.
Hamid Ullah indicated no relevant financial relationships.
Faisal Bukeirat indicated no relevant financial relationships.
Yousef Hreish, MD1, Hamid Ullah, MBBS1, Faisal A.. Bukeirat, MD2. P1786 - Prescriber Beware: Rethinking the Routine Glucagon-Like Peptide-1 Receptor Agonists, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
