Sunday Poster Session
Category: Liver
P1137 - Precision Immunosuppression: Harnessing Mycophenolate Mofetil Monotherapy in a Curated Liver Transplant Population
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E

Has Audio

Ambreen A. Merchant, MD
Baylor University Medical Center
Farmer Branch, TX
Presenting Author(s)
Ambreen Anil Merchant, MD1, Bashar Fteiha, MD2, Soongjin Ahn, DO, MS3, James Trotter, MD4
1Baylor University Medical Center, Farmer Branch, TX; 2Baylor Scott & White Medical Center, Dallas, TX; 3Baylor University, Dallas, TX; 4Intermoutain Health, Murray, UT
Introduction: Historically, monotherapy immunosuppression with cyclosporine, tacrolimus and mTOR-inhibitors (mTORi) have been successfully used individually in liver transplant recipients. However, mycophenolate mofetil (MMF) has been used almost exclusively in combination with a CNI (or less commonly with mTORi) and there is very limited data on its use as a monotherapy in liver transplantation. To evaluate the efficacy and safety of Mycophenolate Mofetil (MMF) monotherapy in post-liver transplantation (LTX) patients, with a focus on the reduction of calcineurin-inhibitor (CNI) related complications such as renal insufficiency, and to assess the rate of acute rejection and overall survival in a real-world clinical setting.
Methods: We performed a retrospective observational cohort study on 37 LTX recipients, analyzing data from 1992-2022. Patients who transitioned to MMF monotherapy were selected based on the absence of previous rejection episodes, stable post-transplant course, and no history of autoimmune liver diseases. Exclusion criteria included multi-organ transplantation and insufficient data. The primary outcome of interest was documented rejection and secondary outcomes including graft loss or death.
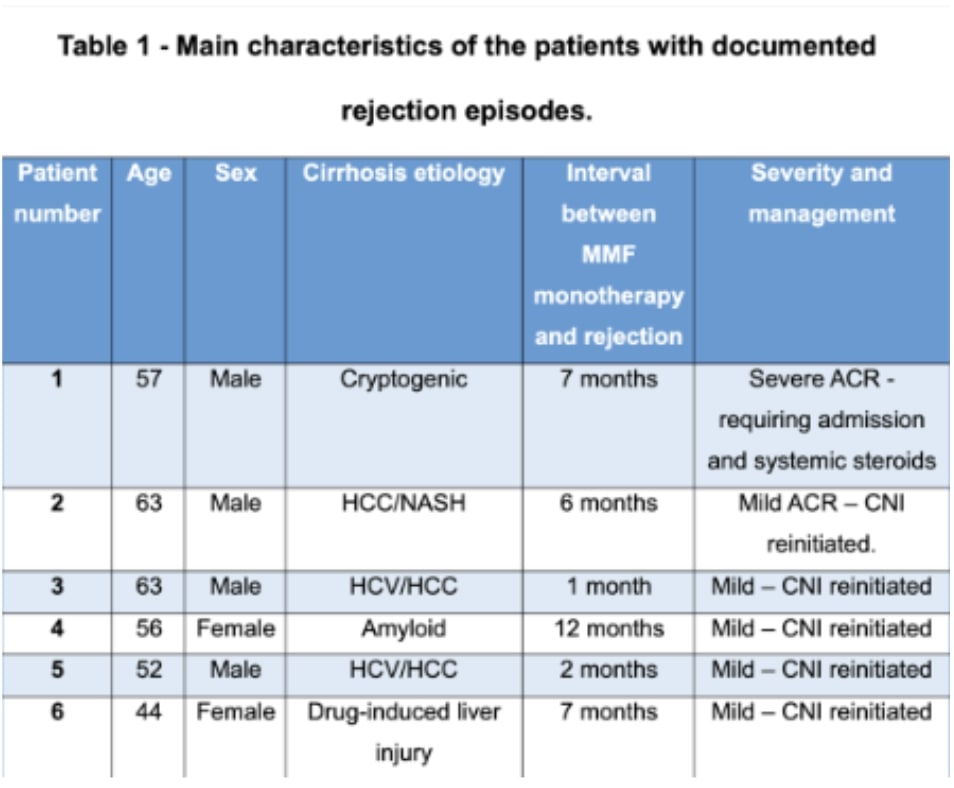
Results: Thirty-three subjects met inclusion criteria and were transitioned to MMF monotherapy post-liver transplantation. Male patients constituted 67.6% (23 subjects), with hepatitis C being the leading indication for transplantation in 38.2% (13 subjects). Hypertension was the most frequent comorbidity, present in 47% (16 subjects), followed by chronic kidney disease in 26.47% (9 subjects). Successful cessation of calcineurin inhibitors was achieved in 81.8% (27 patients), with the transition to MMF occurring at a mean of 84 (± 76) months post-transplant. The follow-up period averaged 8 (± 4) months, during which we noted a mean creatinine reduction of 0.6648 mg/dl (SD = 0.62). Within the follow-up duration, 18.2% (6 patients) experienced acute rejection, with one case severe enough to require hospitalization and pulse steroids, while the remaining cases were managed on an outpatient basis with the reintroduction of CNIs. No cases of graft loss or mortality were observed in our patient cohort.
Discussion: In a carefully selected patient group, MMF monotherapy post-liver transplantation achieved an 81.8% success rate in preserving graft function and reducing CNI-related nephrotoxicity. These promising results advocate for further research to validate this immunosuppressive approach.
Disclosures:
Ambreen Anil Merchant, MD1, Bashar Fteiha, MD2, Soongjin Ahn, DO, MS3, James Trotter, MD4. P1137 - Precision Immunosuppression: Harnessing Mycophenolate Mofetil Monotherapy in a Curated Liver Transplant Population, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Baylor University Medical Center, Farmer Branch, TX; 2Baylor Scott & White Medical Center, Dallas, TX; 3Baylor University, Dallas, TX; 4Intermoutain Health, Murray, UT
Introduction: Historically, monotherapy immunosuppression with cyclosporine, tacrolimus and mTOR-inhibitors (mTORi) have been successfully used individually in liver transplant recipients. However, mycophenolate mofetil (MMF) has been used almost exclusively in combination with a CNI (or less commonly with mTORi) and there is very limited data on its use as a monotherapy in liver transplantation. To evaluate the efficacy and safety of Mycophenolate Mofetil (MMF) monotherapy in post-liver transplantation (LTX) patients, with a focus on the reduction of calcineurin-inhibitor (CNI) related complications such as renal insufficiency, and to assess the rate of acute rejection and overall survival in a real-world clinical setting.
Methods: We performed a retrospective observational cohort study on 37 LTX recipients, analyzing data from 1992-2022. Patients who transitioned to MMF monotherapy were selected based on the absence of previous rejection episodes, stable post-transplant course, and no history of autoimmune liver diseases. Exclusion criteria included multi-organ transplantation and insufficient data. The primary outcome of interest was documented rejection and secondary outcomes including graft loss or death.
Results: Thirty-three subjects met inclusion criteria and were transitioned to MMF monotherapy post-liver transplantation. Male patients constituted 67.6% (23 subjects), with hepatitis C being the leading indication for transplantation in 38.2% (13 subjects). Hypertension was the most frequent comorbidity, present in 47% (16 subjects), followed by chronic kidney disease in 26.47% (9 subjects). Successful cessation of calcineurin inhibitors was achieved in 81.8% (27 patients), with the transition to MMF occurring at a mean of 84 (± 76) months post-transplant. The follow-up period averaged 8 (± 4) months, during which we noted a mean creatinine reduction of 0.6648 mg/dl (SD = 0.62). Within the follow-up duration, 18.2% (6 patients) experienced acute rejection, with one case severe enough to require hospitalization and pulse steroids, while the remaining cases were managed on an outpatient basis with the reintroduction of CNIs. No cases of graft loss or mortality were observed in our patient cohort.
Discussion: In a carefully selected patient group, MMF monotherapy post-liver transplantation achieved an 81.8% success rate in preserving graft function and reducing CNI-related nephrotoxicity. These promising results advocate for further research to validate this immunosuppressive approach.

Figure: Table 1 - Main characteristics of the patients with documented rejection episodes.
Disclosures:
Ambreen Anil Merchant indicated no relevant financial relationships.
Bashar Fteiha indicated no relevant financial relationships.
Soongjin Ahn indicated no relevant financial relationships.
James Trotter indicated no relevant financial relationships.
Ambreen Anil Merchant, MD1, Bashar Fteiha, MD2, Soongjin Ahn, DO, MS3, James Trotter, MD4. P1137 - Precision Immunosuppression: Harnessing Mycophenolate Mofetil Monotherapy in a Curated Liver Transplant Population, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
