Sunday Poster Session
Category: IBD
P0918 - Comparative Efficacy and Safety of Subcutaneous Vedolizumab versus Other Targeted Inflammatory Bowel Disease Therapies in Patients With Moderate to Severe Ulcerative Colitis: A Network Meta-Analysis
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E
Has Audio

Abigail M. Wojtowicz, PhD
Takeda
Cambridge, MA
Presenting Author(s)
Jeanne Jiang, MS1, Abigail M.. Wojtowicz, PhD2, Omolara Sanni, PhD, MPH3, Jennifer Uyei, PhD, MPH3, He Jin, MS3, Kristina Lindsley, PhD3, Marie Sanchirico, MD, PhD4, Tao Fan, MS, PhD1
1Takeda Pharmaceuticals U.S.A., Inc., Cambridge, MA; 2Takeda, Cambridge, MA; 3IQVIA, San Francisco, CA; 4Takeda Pharmaceuticals, USA, Inc., Cambridge, MA
Introduction: The relative efficacy and safety of targeted inflammatory bowel disease (IBD) therapies for treating ulcerative colitis (UC) were assessed in a network meta-analysis (NMA), published in 2021. With the approval of subcutaneous (SC) vedolizumab for the treatment of moderate to severe UC in the USA, we present an updated NMA focused on comparisons of maintenance efficacy and safety outcomes between vedolizumab SC and other targeted IBD therapies.
Methods: A Bayesian NMA was performed using studies identified by a systematic literature search conducted on March 1, 2018, and updated on April 19, 2023. Phase 3, randomized controlled trials of targeted IBD therapies (adalimumab, etrasimod, golimumab, infliximab, mirikizumab, ozanimod, tofacitinib, upadacitinib, ustekinumab and vedolizumab) in adults with moderate to severe UC were included. Outcomes included clinical response and clinical remission during maintenance, overall adverse events (AEs), serious AEs, overall infections, serious infections, and discontinuation due to AEs. Clinical response and remission were as defined by each clinical trial. Fixed effects models were used to compare vedolizumab SC with other treatments and placebo. For efficacy outcomes, the overall population was stratified into anti-tumor necrosis factor α (TNFα)-naïve and anti-TNFα-experienced populations.
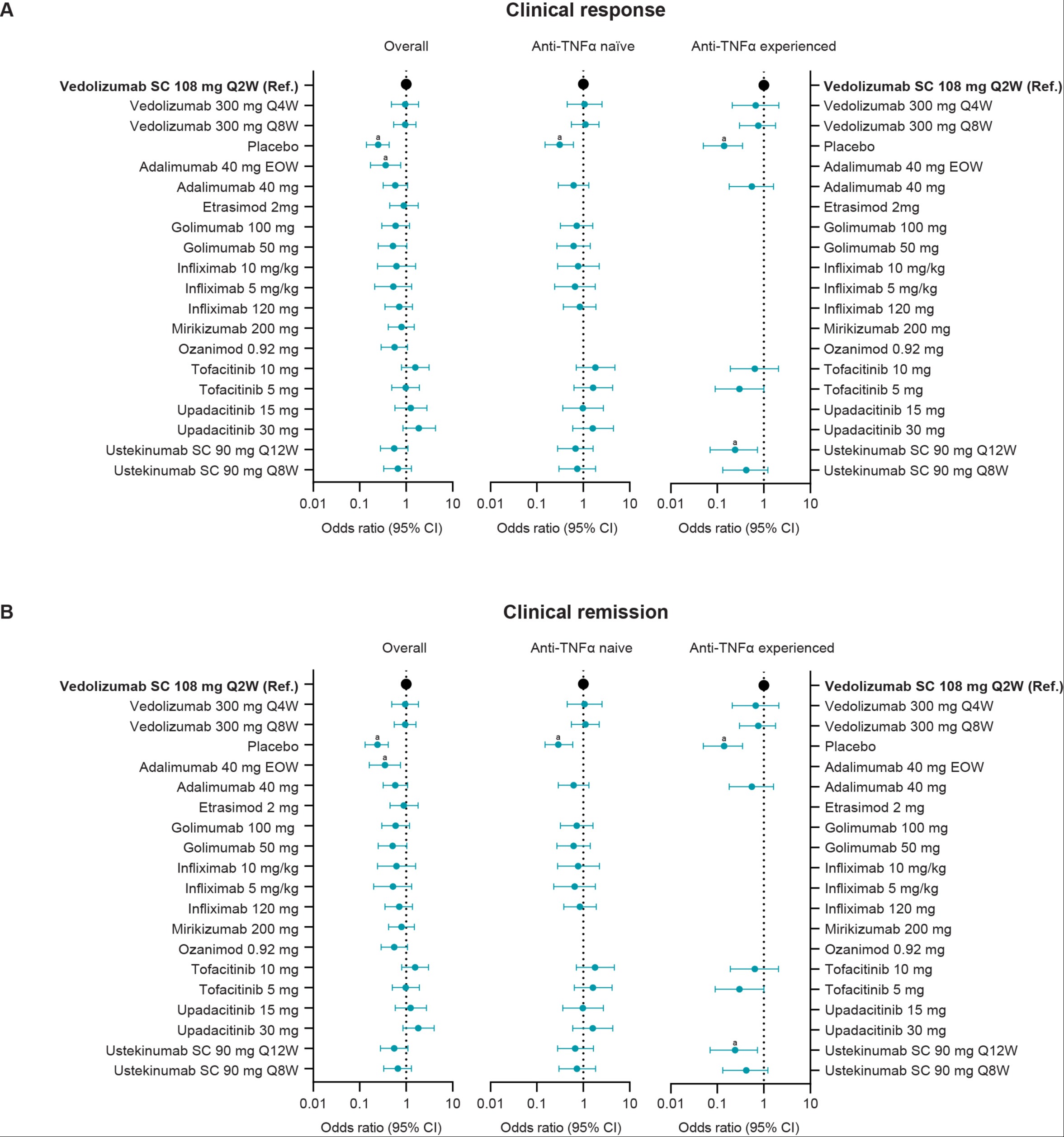
Results: Of the 29 studies, 10 were newly included in this update. The odds of achieving clinical response or clinical remission with vedolizumab SC were either higher or not significantly different to other targeted IBD therapies (Figure 1). For most safety outcomes, except serious infections, vedolizumab SC had a similar or favorable profile compared with other targeted IBD therapies; there were no significant differences between vedolizumab SC and vedolizumab IV (Table 1). Serious infections could not be evaluated due to the low sample size. Vedolizumab SC had significantly lower odds of overall AEs than nine other targeted IBD therapies, and significantly lower odds of overall infections than five other targeted IBD therapies (Table 1).
Discussion: In this updated NMA, vedolizumab SC had similar odds of achieving clinical response and clinical remission and demonstrated a favorable safety profile compared with other targeted IBD therapies for the treatment of moderate to severe UC. The safety profiles for vedolizumab SC and vedolizumab IV were similar. These data will help to inform treatment choices for patients with UC.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Jeanne Jiang, MS1, Abigail M.. Wojtowicz, PhD2, Omolara Sanni, PhD, MPH3, Jennifer Uyei, PhD, MPH3, He Jin, MS3, Kristina Lindsley, PhD3, Marie Sanchirico, MD, PhD4, Tao Fan, MS, PhD1. P0918 - Comparative Efficacy and Safety of Subcutaneous Vedolizumab versus Other Targeted Inflammatory Bowel Disease Therapies in Patients With Moderate to Severe Ulcerative Colitis: A Network Meta-Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Takeda Pharmaceuticals U.S.A., Inc., Cambridge, MA; 2Takeda, Cambridge, MA; 3IQVIA, San Francisco, CA; 4Takeda Pharmaceuticals, USA, Inc., Cambridge, MA
Introduction: The relative efficacy and safety of targeted inflammatory bowel disease (IBD) therapies for treating ulcerative colitis (UC) were assessed in a network meta-analysis (NMA), published in 2021. With the approval of subcutaneous (SC) vedolizumab for the treatment of moderate to severe UC in the USA, we present an updated NMA focused on comparisons of maintenance efficacy and safety outcomes between vedolizumab SC and other targeted IBD therapies.
Methods: A Bayesian NMA was performed using studies identified by a systematic literature search conducted on March 1, 2018, and updated on April 19, 2023. Phase 3, randomized controlled trials of targeted IBD therapies (adalimumab, etrasimod, golimumab, infliximab, mirikizumab, ozanimod, tofacitinib, upadacitinib, ustekinumab and vedolizumab) in adults with moderate to severe UC were included. Outcomes included clinical response and clinical remission during maintenance, overall adverse events (AEs), serious AEs, overall infections, serious infections, and discontinuation due to AEs. Clinical response and remission were as defined by each clinical trial. Fixed effects models were used to compare vedolizumab SC with other treatments and placebo. For efficacy outcomes, the overall population was stratified into anti-tumor necrosis factor α (TNFα)-naïve and anti-TNFα-experienced populations.
Results: Of the 29 studies, 10 were newly included in this update. The odds of achieving clinical response or clinical remission with vedolizumab SC were either higher or not significantly different to other targeted IBD therapies (Figure 1). For most safety outcomes, except serious infections, vedolizumab SC had a similar or favorable profile compared with other targeted IBD therapies; there were no significant differences between vedolizumab SC and vedolizumab IV (Table 1). Serious infections could not be evaluated due to the low sample size. Vedolizumab SC had significantly lower odds of overall AEs than nine other targeted IBD therapies, and significantly lower odds of overall infections than five other targeted IBD therapies (Table 1).
Discussion: In this updated NMA, vedolizumab SC had similar odds of achieving clinical response and clinical remission and demonstrated a favorable safety profile compared with other targeted IBD therapies for the treatment of moderate to severe UC. The safety profiles for vedolizumab SC and vedolizumab IV were similar. These data will help to inform treatment choices for patients with UC.

Figure: Figure 1. Odds of achieving (A) clinical response or (B) clinical remission during maintenance in the overall, anti-TNFα-naïve, or anti-TNFα-experienced populations.
aSignificantly different to vedolizumab SC. An odds ratio of <1 favors vedolizumab SC.
CI, confidence interval; EOW, every other week; Q2W, every 2 weeks; Q4W, every 4 weeks; Q8W, every 8 weeks; Q12W, every 12 weeks; Ref., reference; SC, subcutaneous; TNF, tumor necrosis factor.
aSignificantly different to vedolizumab SC. An odds ratio of <1 favors vedolizumab SC.
CI, confidence interval; EOW, every other week; Q2W, every 2 weeks; Q4W, every 4 weeks; Q8W, every 8 weeks; Q12W, every 12 weeks; Ref., reference; SC, subcutaneous; TNF, tumor necrosis factor.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Jeanne Jiang: Takeda Pharmaceuticals U.S.A., Inc. – Employee, Stock Options.
Abigail Wojtowicz: Takeda – Employee, Stock Options.
Omolara Sanni: IQVIA – Employee, contracted by Takeda Pharmaceuticals U.S.A., Inc. to conduct this study..
Jennifer Uyei: IQVIA – Employee, contracted by Takeda Pharmaceuticals U.S.A., Inc. to conduct this study..
He Jin: IQVIA – Employee, contracted by Takeda Pharmaceuticals U.S.A., Inc. to conduct this study..
Kristina Lindsley: IQVIA – Employee, contracted by Takeda Pharmaceuticals U.S.A., Inc. to conduct this study..
Marie Sanchirico: Takeda Pharmaceuticals U.S.A., Inc. – Employee, Stock Options.
Tao Fan: Takeda Pharmaceuticals U.S.A., Inc. – Employee, Stock Options.
Jeanne Jiang, MS1, Abigail M.. Wojtowicz, PhD2, Omolara Sanni, PhD, MPH3, Jennifer Uyei, PhD, MPH3, He Jin, MS3, Kristina Lindsley, PhD3, Marie Sanchirico, MD, PhD4, Tao Fan, MS, PhD1. P0918 - Comparative Efficacy and Safety of Subcutaneous Vedolizumab versus Other Targeted Inflammatory Bowel Disease Therapies in Patients With Moderate to Severe Ulcerative Colitis: A Network Meta-Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.

