Sunday Poster Session
Category: IBD
P0921 - The Clinical Course of Inflammatory Bowel Disease in Patients Receiving Cancer Therapy
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E
Has Audio

Cristina M. Natha, MD
University of Texas Health, McGovern Medical School
Houston, TX
Presenting Author(s)
Cristina M. Natha, MD1, Varun Vemulapalli, MD1, Kazi Haque, MD1, Andrew Sullivan, MD2, Sidra Naz, MD, MPH3, Emily Zhou, 4, Jasmine Haydel, MD5, Nina Quirk, MS, MD1, Carolina Colli Cruz, MD6, Yinghong Wang, MD, PhD3
1University of Texas Health, McGovern Medical School, Houston, TX; 2University of Texas Health Science Center, Houston, TX; 3University of Texas MD Anderson Cancer Center, Houston, TX; 4McGovern Medical School at UTHealth, Houston, TX; 5Baylor College of Medicine, Houston, TX; 6MD Anderson Cancer Center, Houston, TX
Introduction: Inflammatory bowel disease (IBD) is characterized by chronic inflammation that increases the risk of both gastrointestinal and extraintestinal manifestations, including malignancy. As there is limited data on the impact of cancer treatments on the clinical course of IBD, this study aims to investigate the risk factors and disease course of GI adverse events (GI AE) in patients with cancer and IBD who received cancer therapies of any type.
Methods: This was a single center, retrospective study including patients with malignancy and IBD who received cancer therapy of any type between 2000 and 2024. We collected data on patient demographics and IBD characteristics and compared the findings between patients who did and did not develop GI AE related to cancer therapy. SPSS 24.0 was used to analyze the data.
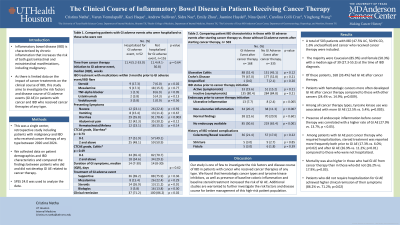
Results: A total of 503 patients with IBD (47.5% UC, 50.9% CD, 1.6% unclassified) and cancer who received cancer therapy were included. The majority were Caucasian (85.9%) and female (50.3%) with a median age of 39 (27.3-55.5) at the time of IBD diagnosis. Of these patients, 168 (33.4%) had GI AE after cancer therapy. Patients with hematologic cancers more often developed GI AE after cancer therapy compared to those with other cancers (24.4% vs. 14.9%; p=0.009). Among all cancer therapy types, tyrosine kinase use was associated with more GI AE (12.5% vs. 5.4%; p=0.005). Presence of endoscopic inflammation before cancer therapy was correlated with higher rate of GI AE (27.9% vs. 13.7; p < 0.05). Among patients with GI AE post cancer therapy who required hospitalization, steroid treatment was reported more frequently both prior to GI AE (17.3% vs. 6.0%; p=0.02) and after GI AE (26.9% vs. 11.2%; p=0.01) compared to those who were not hospitalized. Mortality was also higher in those who had GI AE from cancer therapy than in those who did not (26.2% vs. 17.9%; p=0.03). Patients who did not require hospitalization for GI AE achieved higher clinical remission of their symptoms (86.2% vs. 71.2%; p=0.02).
Discussion: Our study is one of few to investigate the risk factors and disease course of IBD in patients with cancer who received cancer therapies of any type. We found that hematologic cancer type and tyrosine kinase inhibitors, as well as presence of baseline colonic inflammation and baseline steroid treatment increased the risk of GI AE. Additional studies are warranted to further investigate the risk factors and disease course for better management of this high-risk patient population.
Disclosures:
Cristina M. Natha, MD1, Varun Vemulapalli, MD1, Kazi Haque, MD1, Andrew Sullivan, MD2, Sidra Naz, MD, MPH3, Emily Zhou, 4, Jasmine Haydel, MD5, Nina Quirk, MS, MD1, Carolina Colli Cruz, MD6, Yinghong Wang, MD, PhD3. P0921 - The Clinical Course of Inflammatory Bowel Disease in Patients Receiving Cancer Therapy, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1University of Texas Health, McGovern Medical School, Houston, TX; 2University of Texas Health Science Center, Houston, TX; 3University of Texas MD Anderson Cancer Center, Houston, TX; 4McGovern Medical School at UTHealth, Houston, TX; 5Baylor College of Medicine, Houston, TX; 6MD Anderson Cancer Center, Houston, TX
Introduction: Inflammatory bowel disease (IBD) is characterized by chronic inflammation that increases the risk of both gastrointestinal and extraintestinal manifestations, including malignancy. As there is limited data on the impact of cancer treatments on the clinical course of IBD, this study aims to investigate the risk factors and disease course of GI adverse events (GI AE) in patients with cancer and IBD who received cancer therapies of any type.
Methods: This was a single center, retrospective study including patients with malignancy and IBD who received cancer therapy of any type between 2000 and 2024. We collected data on patient demographics and IBD characteristics and compared the findings between patients who did and did not develop GI AE related to cancer therapy. SPSS 24.0 was used to analyze the data.
Results: A total of 503 patients with IBD (47.5% UC, 50.9% CD, 1.6% unclassified) and cancer who received cancer therapy were included. The majority were Caucasian (85.9%) and female (50.3%) with a median age of 39 (27.3-55.5) at the time of IBD diagnosis. Of these patients, 168 (33.4%) had GI AE after cancer therapy. Patients with hematologic cancers more often developed GI AE after cancer therapy compared to those with other cancers (24.4% vs. 14.9%; p=0.009). Among all cancer therapy types, tyrosine kinase use was associated with more GI AE (12.5% vs. 5.4%; p=0.005). Presence of endoscopic inflammation before cancer therapy was correlated with higher rate of GI AE (27.9% vs. 13.7; p < 0.05). Among patients with GI AE post cancer therapy who required hospitalization, steroid treatment was reported more frequently both prior to GI AE (17.3% vs. 6.0%; p=0.02) and after GI AE (26.9% vs. 11.2%; p=0.01) compared to those who were not hospitalized. Mortality was also higher in those who had GI AE from cancer therapy than in those who did not (26.2% vs. 17.9%; p=0.03). Patients who did not require hospitalization for GI AE achieved higher clinical remission of their symptoms (86.2% vs. 71.2%; p=0.02).
Discussion: Our study is one of few to investigate the risk factors and disease course of IBD in patients with cancer who received cancer therapies of any type. We found that hematologic cancer type and tyrosine kinase inhibitors, as well as presence of baseline colonic inflammation and baseline steroid treatment increased the risk of GI AE. Additional studies are warranted to further investigate the risk factors and disease course for better management of this high-risk patient population.
Disclosures:
Cristina Natha indicated no relevant financial relationships.
Varun Vemulapalli indicated no relevant financial relationships.
Kazi Haque indicated no relevant financial relationships.
Andrew Sullivan indicated no relevant financial relationships.
Sidra Naz indicated no relevant financial relationships.
Emily Zhou indicated no relevant financial relationships.
Jasmine Haydel indicated no relevant financial relationships.
Nina Quirk indicated no relevant financial relationships.
Carolina Colli Cruz indicated no relevant financial relationships.
Yinghong Wang: AzurRx – Consultant. Ilyapharma – Consultant. IOTA – Consultant. Sorriso – Consultant. Tillotts – Consultant.
Cristina M. Natha, MD1, Varun Vemulapalli, MD1, Kazi Haque, MD1, Andrew Sullivan, MD2, Sidra Naz, MD, MPH3, Emily Zhou, 4, Jasmine Haydel, MD5, Nina Quirk, MS, MD1, Carolina Colli Cruz, MD6, Yinghong Wang, MD, PhD3. P0921 - The Clinical Course of Inflammatory Bowel Disease in Patients Receiving Cancer Therapy, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
