Sunday Poster Session
Category: IBD
P0923 - Female Sex Associated with Decreased Time to Disease-Related Complication Following Biologic Initiation in Biologic-Naive Patients With Ulcerative Colitis
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E
Has Audio

Saihej P. Basra
Feinberg School of Medicine, Northwestern University
Chicago, IL
Presenting Author(s)
Saihej P. Basra, 1, Matthew B. Stanton, MD2, Stephen B. Hanauer, MD, FACG3, Parambir S. Dulai, MD1
1Feinberg School of Medicine, Northwestern University, Chicago, IL; 2Northwestern University, Chicago, IL; 3Northwestern Medicine, Chicago, IL
Introduction: Previous studies analyzing sex differences in inflammatory bowel disease (IBD) have found that females are less likely to adhere to biologics and more likely to have side effects. This study aims to analyze the relationship between sex and time to disease-related complication after biologic initiation in biologic-naïve patients with ulcerative colitis (UC).
Methods: This study analyzed interim data from a retrospective chart review of IBD patients started on a biologic from 2018 to present at an integrated academic health network of 11 hospitals. This analysis included 170 biologic-naïve patients diagnosed with UC. Disease-related complications were defined as emergency department (ED) visits, hospitalizations, or bowel surgeries due to UC. Time to complication was defined as the number of days from biologic initiation to the patient’s first subsequent complication. For patients with no complications during the study period, time to complication was defined as the number of days from biologic initiation to their latest IBD office visit. Mean time to complication was compared between males and females. Additionally, the rate of complications was compared between males and females. The Cox proportional hazards model was used to analyze the independent impact of sex on time to complication.
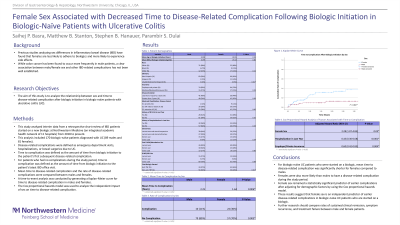
Results: The 170 patients in this study included 89 males and 81 females. 44% had a prior ED visit for UC, 49% had a prior hospitalization for UC, and 19% had Medicare/Medicaid/no insurance. 46% were started on an anti-tumor necrosis factor (anti-TNF) biologic while 54% were started on a non-anti-TNF biologic. Mean time to disease-related complication was 600 days for females and 820 days for males (p=0.006). 30% of females had a complication during the study period, compared to 11% of males (p=0.003). After adjusting for demographic factors, female sex was associated with an increased risk of disease-related complication (adjusted hazard ratio, 2.28; 95% confidence interval, 1.07-4.85).
Discussion: For biologic-naïve UC patients who were started on a biologic, mean time to disease-related complication was significantly shorter for females compared to males. Females were more likely to have a disease-related complication during the study period. Female sex remained a statistically significant predictor of earlier complications after adjusting for demographic factors. These results suggest that female sex is an independent predictor of earlier complications after biologic initiation in patients with UC.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Saihej P. Basra, 1, Matthew B. Stanton, MD2, Stephen B. Hanauer, MD, FACG3, Parambir S. Dulai, MD1. P0923 - Female Sex Associated with Decreased Time to Disease-Related Complication Following Biologic Initiation in Biologic-Naive Patients With Ulcerative Colitis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Feinberg School of Medicine, Northwestern University, Chicago, IL; 2Northwestern University, Chicago, IL; 3Northwestern Medicine, Chicago, IL
Introduction: Previous studies analyzing sex differences in inflammatory bowel disease (IBD) have found that females are less likely to adhere to biologics and more likely to have side effects. This study aims to analyze the relationship between sex and time to disease-related complication after biologic initiation in biologic-naïve patients with ulcerative colitis (UC).
Methods: This study analyzed interim data from a retrospective chart review of IBD patients started on a biologic from 2018 to present at an integrated academic health network of 11 hospitals. This analysis included 170 biologic-naïve patients diagnosed with UC. Disease-related complications were defined as emergency department (ED) visits, hospitalizations, or bowel surgeries due to UC. Time to complication was defined as the number of days from biologic initiation to the patient’s first subsequent complication. For patients with no complications during the study period, time to complication was defined as the number of days from biologic initiation to their latest IBD office visit. Mean time to complication was compared between males and females. Additionally, the rate of complications was compared between males and females. The Cox proportional hazards model was used to analyze the independent impact of sex on time to complication.
Results: The 170 patients in this study included 89 males and 81 females. 44% had a prior ED visit for UC, 49% had a prior hospitalization for UC, and 19% had Medicare/Medicaid/no insurance. 46% were started on an anti-tumor necrosis factor (anti-TNF) biologic while 54% were started on a non-anti-TNF biologic. Mean time to disease-related complication was 600 days for females and 820 days for males (p=0.006). 30% of females had a complication during the study period, compared to 11% of males (p=0.003). After adjusting for demographic factors, female sex was associated with an increased risk of disease-related complication (adjusted hazard ratio, 2.28; 95% confidence interval, 1.07-4.85).
Discussion: For biologic-naïve UC patients who were started on a biologic, mean time to disease-related complication was significantly shorter for females compared to males. Females were more likely to have a disease-related complication during the study period. Female sex remained a statistically significant predictor of earlier complications after adjusting for demographic factors. These results suggest that female sex is an independent predictor of earlier complications after biologic initiation in patients with UC.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Saihej Basra indicated no relevant financial relationships.
Matthew Stanton indicated no relevant financial relationships.
Stephen B. Hanauer: Abbvie – Consultant. Johnson and Johnson – Consultant. Takeda – Consultant.
Parambir Dulai: AbbVie – Consultant. Abivax – Consultant. Adiso – Consultant. Bristol Meyer Squibb – Consultant. Digbi Health – Royalties. Digbi Health – Stock Options. Geneoscopy – Consultant. GSK – Consultant. Janssen – Consultant. Lilly – Consultant. Pfizer – Consultant, Grant/Research Support. Precidiag – Licensing royalties. Takeda – Consultant, Grant/Research Support.
Saihej P. Basra, 1, Matthew B. Stanton, MD2, Stephen B. Hanauer, MD, FACG3, Parambir S. Dulai, MD1. P0923 - Female Sex Associated with Decreased Time to Disease-Related Complication Following Biologic Initiation in Biologic-Naive Patients With Ulcerative Colitis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
