Sunday Poster Session
Category: IBD
P0837 - Evaluating the Use of Machine Learning to Model Nutritional Triggers in Patients With Inflammatory Bowel Disease
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E

Has Audio

Ameya Saraf
University of Cincinnati College of Medicine
Cincinnati, OH
Presenting Author(s)
Ameya Saraf, 1, John Shinn, APN1, Kelsie Newton, RD2, Kara DeFelice, MD1, Emily Scott, PA-C1, Tara Nagaraj, PharmD2, Lee Beck, RN2, Rebecca Crum, RN1, Anita Afzali, MD, MPH, MHCM, FACG1
1University of Cincinnati College of Medicine, Cincinnati, OH; 2University of Cincinnati Medical Center, Cincinnati, OH
Introduction: Despite the well-known impact of nutrition on Inflammatory Bowel Disease (IBD), its role is often underestimated in disease management. Two-thirds of IBD patients restrict their food intake due to intolerance, and identifying what to eliminate from their diet is frequently an arduous process involving much trial and error. Excess dietary restriction may result in malnutrition, a comorbidity highly prevalent among IBD patients. Patients require a personalized and attainable dietary plan. Thus, we sought to employ machine learning to model symptom-causing triggers in IBD patients who are in endoscopic remission but continue to remain with active bowel symptoms.
Methods: Subjects were screened using chart review and over-the-phone interviews. Selected subjects had evidence of endoscopic remission (Mayo or SES-CD score) and were on medical treatment for IBD, yet still experienced recurrent symptoms. 25 subjects downloaded a uniquely designed mobile application created for this study to track diet, upper and lower GI symptoms, bowel movements (frequency and Bristol score), and other lifestyle factors like exercise and sleep, for at least 2 consecutive weeks. Both unsupervised and supervised models were utilized to analyze each patient. Association rule and feature importance analyses were done to identify features contributing most toward target symptoms. Supervised models such as decision trees and support vector machines were constructed from these target features.
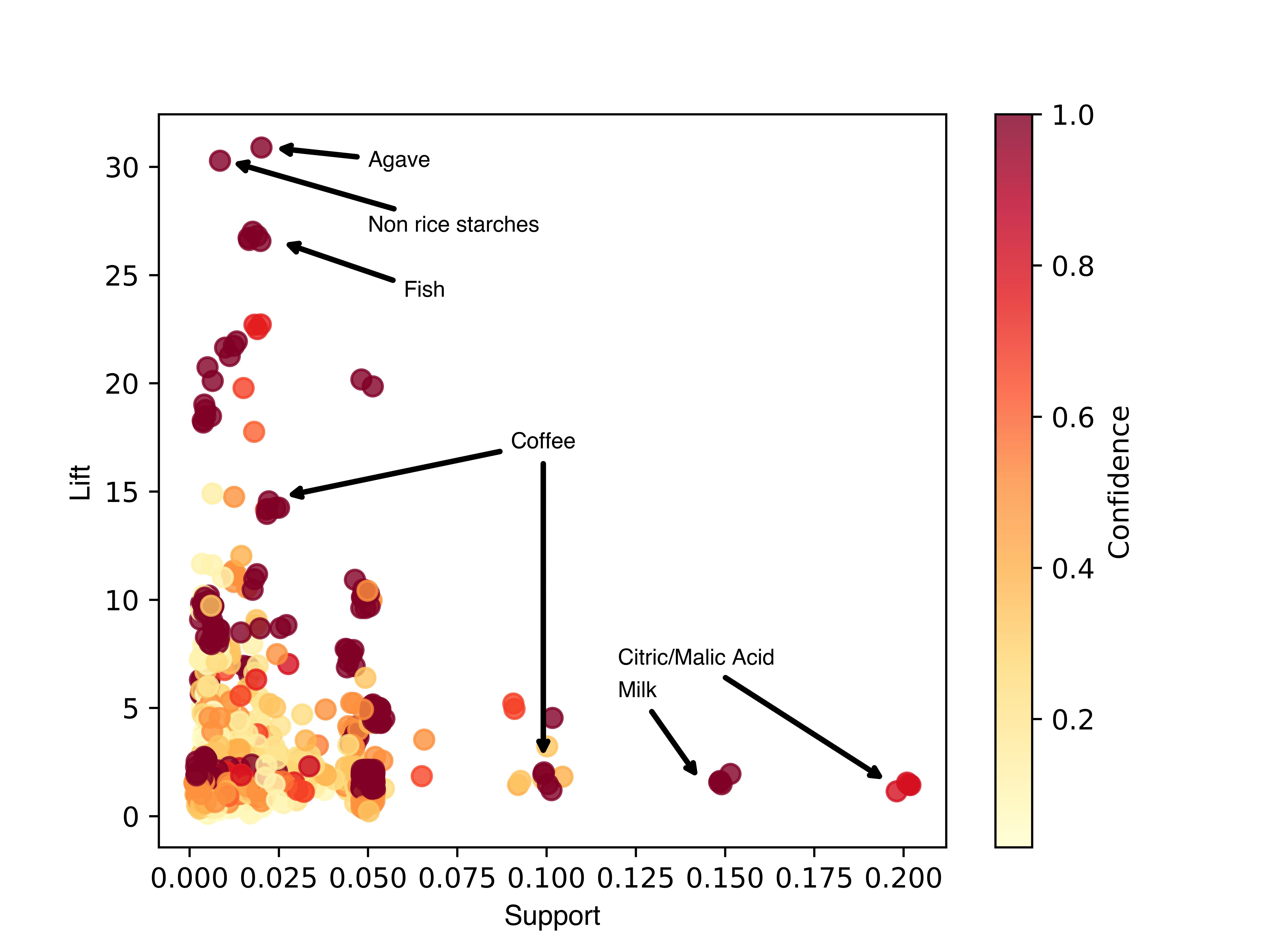
Results: Of the 25 enrolled patients, 14 logged data for at least two weeks. Unsupervised models were able to discern key features causing symptoms. Exercise and sleep were found to have minimal/no impact on symptoms, while mood had a slight effect, and ingredients had the greatest effect. For every patient, unsupervised models detected specific ingredients with high probabilities of being symptom triggers (Figure 1). For patients with enough data, supervised learning models could predict symptoms with >90% accuracy, and high precision and recall per class.
Discussion: Tracking and analyzing data to create patient-specific dietary recommendations can be an invaluable tool for clinicians in managing IBD patients who are in endoscopic remission but continue to report active bowel symptoms. While future studies are necessary to evaluate the efficacy of these models as a clinical intervention, we have demonstrated their viability and have developed an app to efficiently monitor and implement their use in healthcare settings.
Disclosures:
Ameya Saraf, 1, John Shinn, APN1, Kelsie Newton, RD2, Kara DeFelice, MD1, Emily Scott, PA-C1, Tara Nagaraj, PharmD2, Lee Beck, RN2, Rebecca Crum, RN1, Anita Afzali, MD, MPH, MHCM, FACG1. P0837 - Evaluating the Use of Machine Learning to Model Nutritional Triggers in Patients With Inflammatory Bowel Disease, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1University of Cincinnati College of Medicine, Cincinnati, OH; 2University of Cincinnati Medical Center, Cincinnati, OH
Introduction: Despite the well-known impact of nutrition on Inflammatory Bowel Disease (IBD), its role is often underestimated in disease management. Two-thirds of IBD patients restrict their food intake due to intolerance, and identifying what to eliminate from their diet is frequently an arduous process involving much trial and error. Excess dietary restriction may result in malnutrition, a comorbidity highly prevalent among IBD patients. Patients require a personalized and attainable dietary plan. Thus, we sought to employ machine learning to model symptom-causing triggers in IBD patients who are in endoscopic remission but continue to remain with active bowel symptoms.
Methods: Subjects were screened using chart review and over-the-phone interviews. Selected subjects had evidence of endoscopic remission (Mayo or SES-CD score) and were on medical treatment for IBD, yet still experienced recurrent symptoms. 25 subjects downloaded a uniquely designed mobile application created for this study to track diet, upper and lower GI symptoms, bowel movements (frequency and Bristol score), and other lifestyle factors like exercise and sleep, for at least 2 consecutive weeks. Both unsupervised and supervised models were utilized to analyze each patient. Association rule and feature importance analyses were done to identify features contributing most toward target symptoms. Supervised models such as decision trees and support vector machines were constructed from these target features.
Results: Of the 25 enrolled patients, 14 logged data for at least two weeks. Unsupervised models were able to discern key features causing symptoms. Exercise and sleep were found to have minimal/no impact on symptoms, while mood had a slight effect, and ingredients had the greatest effect. For every patient, unsupervised models detected specific ingredients with high probabilities of being symptom triggers (Figure 1). For patients with enough data, supervised learning models could predict symptoms with >90% accuracy, and high precision and recall per class.
Discussion: Tracking and analyzing data to create patient-specific dietary recommendations can be an invaluable tool for clinicians in managing IBD patients who are in endoscopic remission but continue to report active bowel symptoms. While future studies are necessary to evaluate the efficacy of these models as a clinical intervention, we have demonstrated their viability and have developed an app to efficiently monitor and implement their use in healthcare settings.

Figure: Figure 1. Ingredient-Symptom Association Rule Metrics for all Subjects. Association rules identified key symptom-contributing ingredients. A priori analysis was conducted for every subject and plotted according to support (frequency of the ingredient and symptom appearing together), lift (increase in the probability of having the symptom after eating an ingredient), and confidence (likelihood of having a symptom after an ingredient is consumed). Datapoints represent rules between ingredients and symptoms. Notable ingredients are marked.
Disclosures:
Ameya Saraf indicated no relevant financial relationships.
John Shinn indicated no relevant financial relationships.
Kelsie Newton indicated no relevant financial relationships.
Kara DeFelice: Abbvie – Advisory Committee/Board Member, Speakers Bureau. BMS – Advisory Committee/Board Member, Speakers Bureau. Janssen – Honorary Speaker.
Emily Scott indicated no relevant financial relationships.
Tara Nagaraj indicated no relevant financial relationships.
Lee Beck indicated no relevant financial relationships.
Rebecca Crum indicated no relevant financial relationships.
Anita Afzali: AbbVie – Consultant. Bristol Myers Squibb/Celgene – Consultant. Cullgen – Consultant. DiaSorin – Consultant. Eli Lilly – Consultant. Gilead – Consultant. IBD Horizons – Consultant. Janssen – Consultant. Pfizer – Consultant. Takeda – Consultant. TLL Pharmaceuticals – Consultant.
Ameya Saraf, 1, John Shinn, APN1, Kelsie Newton, RD2, Kara DeFelice, MD1, Emily Scott, PA-C1, Tara Nagaraj, PharmD2, Lee Beck, RN2, Rebecca Crum, RN1, Anita Afzali, MD, MPH, MHCM, FACG1. P0837 - Evaluating the Use of Machine Learning to Model Nutritional Triggers in Patients With Inflammatory Bowel Disease, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
