Sunday Poster Session
Category: IBD
P0838 - Early Observations in the Real-World Use of Risankizumab Therapy for Crohn’s Disease
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E
Has Audio

Anna Leone, BS
The Ohio State University College of Medicine
Columbus, OH
Presenting Author(s)
Anna Leone, BS1, Alyssa Drosdak, MD2, Adeeti Chiplunker, MD2
1The Ohio State University College of Medicine, Columbus, OH; 2The Ohio State University Wexner Medical Center, Columbus, OH
Introduction: Risankizumab (RZA), a monoclonal antibody that inhibits the p-19 subunit of IL-23, was first approved for treatment of moderate to severe Crohn’s disease in June 2022. We present the early single-center experience of RZA use in Crohn’s disease patients at The Ohio State University Inflammatory Bowel Disease Center (OSU IBD).
Methods: The study cohort included patients started on RZA at the OSU IBD Center. Patients were excluded if they started on RZA at another institution. Electronic medical records were reviewed from April 2022 - May 2024 to collect patient demographics (age, race, ethnicity), BMI, and disease characteristics (distribution, phenotype, duration, prior treatment failure with anti-TNF, prior treatment failure with Ustekinumab). Measures of disease activity were obtained prior to RZA initiation and during therapy: liquid stool count, Harvey Bradshaw Index score, Simple Endoscopic Score on endoscopic exam, serum C-reactive protein (CRP), and fecal calprotectin. T-test was used to evaluate continuous variables, and Fisher’s exact test was used for categorical variables.
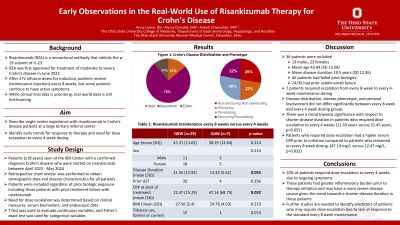
Results: 38 patients began RZA during this period, and 36 were included in the study (mean age 44, 64% female). Disease distribution included ileocolonic (n=27), ileal (n=5), and colonic (n=4). Disease phenotype varied: 10 stricturing/non-penetrating, 8 stricturing, 10 penetrating, and 8 stricturing/penetrating disease. 15 patients also had perianal disease. Mean disease duration prior to RZA induction was 19.5 years. All patients had prior biologic failure, and all but one patient had prior Infliximab failure. 24 patients experienced prior Ustekinumab failure. 7 patients escalated from every 8-week to every 4-week 360mg RZA dosing after inadequate response by clinical measures of disease activity including symptoms and biomarker levels. Patients who were increased to every 4-week dosing had significantly shorter disease duration (mean 11.43 years versus 21.45 years, p=0.055), but were similar in age (38.29 years-old versus 45.31 years old, p=0.214). Patients started on 4-week dosing had higher serum CRP prior to initiation of RZA (47.14 mg/L versus 12.47 mg/L, p=0.032).
Discussion: Among patients who started RZA therapy, a significant portion did not respond to standard dosing. Approximately 20 percent required dose-escalation to every 4-week administration. These patients had greater inflammatory burden prior to initiation shown by higher serum CRP.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Anna Leone, BS1, Alyssa Drosdak, MD2, Adeeti Chiplunker, MD2. P0838 - Early Observations in the Real-World Use of Risankizumab Therapy for Crohn’s Disease, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1The Ohio State University College of Medicine, Columbus, OH; 2The Ohio State University Wexner Medical Center, Columbus, OH
Introduction: Risankizumab (RZA), a monoclonal antibody that inhibits the p-19 subunit of IL-23, was first approved for treatment of moderate to severe Crohn’s disease in June 2022. We present the early single-center experience of RZA use in Crohn’s disease patients at The Ohio State University Inflammatory Bowel Disease Center (OSU IBD).
Methods: The study cohort included patients started on RZA at the OSU IBD Center. Patients were excluded if they started on RZA at another institution. Electronic medical records were reviewed from April 2022 - May 2024 to collect patient demographics (age, race, ethnicity), BMI, and disease characteristics (distribution, phenotype, duration, prior treatment failure with anti-TNF, prior treatment failure with Ustekinumab). Measures of disease activity were obtained prior to RZA initiation and during therapy: liquid stool count, Harvey Bradshaw Index score, Simple Endoscopic Score on endoscopic exam, serum C-reactive protein (CRP), and fecal calprotectin. T-test was used to evaluate continuous variables, and Fisher’s exact test was used for categorical variables.
Results: 38 patients began RZA during this period, and 36 were included in the study (mean age 44, 64% female). Disease distribution included ileocolonic (n=27), ileal (n=5), and colonic (n=4). Disease phenotype varied: 10 stricturing/non-penetrating, 8 stricturing, 10 penetrating, and 8 stricturing/penetrating disease. 15 patients also had perianal disease. Mean disease duration prior to RZA induction was 19.5 years. All patients had prior biologic failure, and all but one patient had prior Infliximab failure. 24 patients experienced prior Ustekinumab failure. 7 patients escalated from every 8-week to every 4-week 360mg RZA dosing after inadequate response by clinical measures of disease activity including symptoms and biomarker levels. Patients who were increased to every 4-week dosing had significantly shorter disease duration (mean 11.43 years versus 21.45 years, p=0.055), but were similar in age (38.29 years-old versus 45.31 years old, p=0.214). Patients started on 4-week dosing had higher serum CRP prior to initiation of RZA (47.14 mg/L versus 12.47 mg/L, p=0.032).
Discussion: Among patients who started RZA therapy, a significant portion did not respond to standard dosing. Approximately 20 percent required dose-escalation to every 4-week administration. These patients had greater inflammatory burden prior to initiation shown by higher serum CRP.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Anna Leone indicated no relevant financial relationships.
Alyssa Drosdak indicated no relevant financial relationships.
Adeeti Chiplunker: Janssen Biotech, Inc – Advisor or Review Panel Member.
Anna Leone, BS1, Alyssa Drosdak, MD2, Adeeti Chiplunker, MD2. P0838 - Early Observations in the Real-World Use of Risankizumab Therapy for Crohn’s Disease, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
