Sunday Poster Session
Category: Colon
P0228 - Impact of Transitioning From PCR-Only to Multi-Step Clostridioides difficile Testing in a Large Hospital System
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E
Has Audio
- TS
Tyler M. Selig, MD
Warren Alpert Medical School of Brown University
Stony Brook, NY
Presenting Author(s)
Tyler M. Selig, MD1, Kathryn Evey, DO1, Adam Burton, MD1, Sapana R. Gupta, MD2, Curtis Petruzzelli, MD2, Jacqueline Chu, MD1, Wen Ting Yang, MD2, Michael Rossi, MD1, Joshua Ray. Tanzer, PhD3, John R. Lonks, MD4, Colleen R. Kelly, MD, FACG5
1Warren Alpert Medical School of Brown University, Providence, RI; 2Brown University / Rhode Island Hospital, Providence, RI; 3Rhode Island Hospital, Providence, RI; 4The Miriam Hospital, Providence, RI; 5Brigham and Women's Hospital, Harvard Medical School, Boston, MA
Introduction: The toxin enzyme immunoassay (EIA) for <em>C. difficile</em> detects the presence of free toxin in the stool, and while specific for <em>C. difficile</em> infection (CDI), sensitivity is low. Highly sensitive nucleic acid amplification testing (NAAT), such as polymerase chain reaction (PCR), tests for the presence of a toxigenic strain of the organism but cannot differentiate between infection and colonization. Infectious Disease Society guidelines recommend NAAT may be used as a stand-alone test, while American College of Gastroenterology guidelines recommend a 2-step algorithm, utilizing both test types, for best diagnostic accuracy. In June 2021, Rhode Island’s largest hospital system changed from using PCR only testing to a 2-step process, starting with PCR and, when positive, reflexing to toxin EIA confirmatory testing. The primary purpose of this study was to determine whether the implementation of 2-step testing led to adverse patient outcomes.
Methods: This is a retrospective cohort study of 2,173 adult inpatients at two tertiary care hospitals who had testing for CDI. Patients were divided into two groups: those tested for toxigenic <em>C. difficile</em> via PCR-only between June 2019 and May 2021 (n = 1194) and those who were tested using the 2-step algorithm between June 2021 and May 2023 (n = 979). Cluster analysis was used to identify appropriate patient risk groups for hypothesis generation, and the frequency of complications including death, colectomy, transfer to an intensive care unit (ICU), and 30-day readmission were compared within these groups.
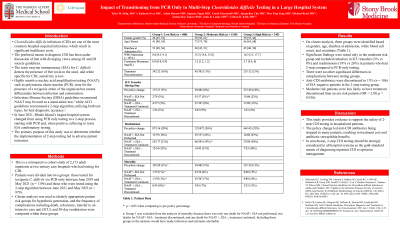
Results: On cluster analysis, three groups were identified based on gender, age, diarrhea at admission, white blood cell count, and creatinine (Table 1). Significant findings were noted only in the moderate risk group and included reduction in ICU transfers (3% vs 8%) and readmissions (19% vs 26%) in patients who had 2-step compared to PCR-only testing. There were no other significant differences in complications between testing groups. Anti-CDI antibiotics were discontinued in 15% (n = 106) of EIA negative patients in the 2-step testing group. Moderate risk patients were less likely to have treatment discontinued than severe risk patients (OR = 2.00, p = 0.016).
Discussion: This study provides evidence to support the safety of 2-step CDI testing in hospitalized patients. The policy change led anti-CDI antibiotics being stopped in many patients, resulting in treatment cost and antibiotic stewardship benefits.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Tyler M. Selig, MD1, Kathryn Evey, DO1, Adam Burton, MD1, Sapana R. Gupta, MD2, Curtis Petruzzelli, MD2, Jacqueline Chu, MD1, Wen Ting Yang, MD2, Michael Rossi, MD1, Joshua Ray. Tanzer, PhD3, John R. Lonks, MD4, Colleen R. Kelly, MD, FACG5. P0228 - Impact of Transitioning From PCR-Only to Multi-Step <i>Clostridioides difficile</i> Testing in a Large Hospital System, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Warren Alpert Medical School of Brown University, Providence, RI; 2Brown University / Rhode Island Hospital, Providence, RI; 3Rhode Island Hospital, Providence, RI; 4The Miriam Hospital, Providence, RI; 5Brigham and Women's Hospital, Harvard Medical School, Boston, MA
Introduction: The toxin enzyme immunoassay (EIA) for <em>C. difficile</em> detects the presence of free toxin in the stool, and while specific for <em>C. difficile</em> infection (CDI), sensitivity is low. Highly sensitive nucleic acid amplification testing (NAAT), such as polymerase chain reaction (PCR), tests for the presence of a toxigenic strain of the organism but cannot differentiate between infection and colonization. Infectious Disease Society guidelines recommend NAAT may be used as a stand-alone test, while American College of Gastroenterology guidelines recommend a 2-step algorithm, utilizing both test types, for best diagnostic accuracy. In June 2021, Rhode Island’s largest hospital system changed from using PCR only testing to a 2-step process, starting with PCR and, when positive, reflexing to toxin EIA confirmatory testing. The primary purpose of this study was to determine whether the implementation of 2-step testing led to adverse patient outcomes.
Methods: This is a retrospective cohort study of 2,173 adult inpatients at two tertiary care hospitals who had testing for CDI. Patients were divided into two groups: those tested for toxigenic <em>C. difficile</em> via PCR-only between June 2019 and May 2021 (n = 1194) and those who were tested using the 2-step algorithm between June 2021 and May 2023 (n = 979). Cluster analysis was used to identify appropriate patient risk groups for hypothesis generation, and the frequency of complications including death, colectomy, transfer to an intensive care unit (ICU), and 30-day readmission were compared within these groups.
Results: On cluster analysis, three groups were identified based on gender, age, diarrhea at admission, white blood cell count, and creatinine (Table 1). Significant findings were noted only in the moderate risk group and included reduction in ICU transfers (3% vs 8%) and readmissions (19% vs 26%) in patients who had 2-step compared to PCR-only testing. There were no other significant differences in complications between testing groups. Anti-CDI antibiotics were discontinued in 15% (n = 106) of EIA negative patients in the 2-step testing group. Moderate risk patients were less likely to have treatment discontinued than severe risk patients (OR = 2.00, p = 0.016).
Discussion: This study provides evidence to support the safety of 2-step CDI testing in hospitalized patients. The policy change led anti-CDI antibiotics being stopped in many patients, resulting in treatment cost and antibiotic stewardship benefits.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Tyler Selig indicated no relevant financial relationships.
Kathryn Evey indicated no relevant financial relationships.
Adam Burton indicated no relevant financial relationships.
Sapana Gupta indicated no relevant financial relationships.
Curtis Petruzzelli indicated no relevant financial relationships.
Jacqueline Chu indicated no relevant financial relationships.
Wen Ting Yang indicated no relevant financial relationships.
Michael Rossi indicated no relevant financial relationships.
Joshua Tanzer indicated no relevant financial relationships.
John Lonks indicated no relevant financial relationships.
Colleen Kelly indicated no relevant financial relationships.
Tyler M. Selig, MD1, Kathryn Evey, DO1, Adam Burton, MD1, Sapana R. Gupta, MD2, Curtis Petruzzelli, MD2, Jacqueline Chu, MD1, Wen Ting Yang, MD2, Michael Rossi, MD1, Joshua Ray. Tanzer, PhD3, John R. Lonks, MD4, Colleen R. Kelly, MD, FACG5. P0228 - Impact of Transitioning From PCR-Only to Multi-Step <i>Clostridioides difficile</i> Testing in a Large Hospital System, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
