Sunday Poster Session
Category: Colon
P0229 - Impact of Patient and Provider Variability on Compliance With C. difficile Testing for IBD Flares
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E
Has Audio
.jpg)
Danielle Garfunkel, MD
Montefiore Medical Center
Scarsdale, NY
Presenting Author(s)
Danielle Garfunkel, MD1, Daniel Alvarez, MD2, George G.. Papadopoulos, MD3, Hamza Sadhra, MD3, Melissa Fazzari, PhD, MS4, Ruby Greywoode, MD, MS4, Thomas Ullman, MD4
1Montefiore Medical Center, Scarsdale, NY; 2Montefiore Medical Center/Albert Einstein College of Medicine, Bronx, NY; 3Montefiore Medical Center, Bronx, NY; 4Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, NY
Introduction: Over three decades, the incidence of Clostridioides difficile (C-diff) in inflammatory bowel disease (IBD) patients has risen dramatically. Though guidelines advise testing all patients with IBD flares for C-diff, there is little data on compliance with this recommendation. This study investigates C-diff testing compliance rates, factors affecting testing and impact of testing in IBD flares on length of stay (LOS) and readmissions.
Methods: We conducted a retrospective cohort study at a tertiary academic medical center of adults admitted for IBD flares with documented diarrhea from 3/2019-3/2021. We excluded patients who left the emergency department (ED) against medical advice. Demographic and clinical data were gathered by chart review. Primary outcome was C-diff testing rates. Secondary outcomes included time to testing. Admissions from 3/2019-3/2020 were compared to 3/2020-3/2021 to evaluate the COVID pandemic effect. We employed chi-square and Wilcoxon Mann-Whitney tests to examine associations and multivariate Poisson regression model to adjust for confounders.
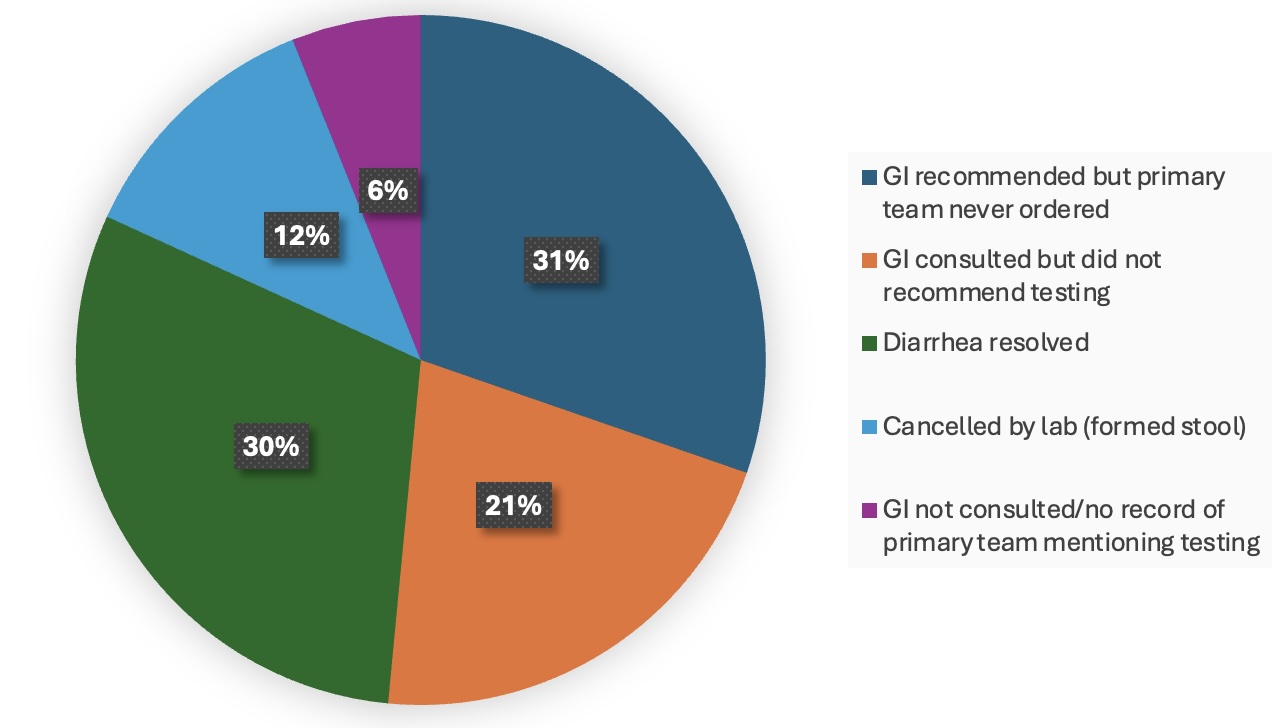
Results: Of 802 admissions, 195 (24%) were included. The mean age was 38 years with 55% males, 55% of Hispanic ethnicity and 53% with ulcerative colitis (Table 1). 83% of cases were tested for C-diff with a 7% positivity rate. 71% were sent in the inpatient setting rather than the ED and 66% were sent before day 2 of hospitalization. Of those not tested, 30% had resolved diarrhea and 12% were canceled by lab (Fig. 1). Factors associated with increased testing included gastroenterology consultation (85% vs 63%, p=0.02), higher heart rate (98 vs 87, p=0.02), and positive SIRS criteria (91% vs 79%, p=0.04). During the COVID pandemic, ED testing rates declined (38% vs 18%, p=0.01). After adjusting for confounders, expected LOS was 54% longer among those tested than not tested (p< 0.0001). Those tested tended to have longer time to readmission (104d vs 45d, p=0.19).
Discussion: At our hospital, C-diff testing rates for IBD flares were relatively high, though most tests were delayed until inpatient admission. Gastroenterology consultation and patient illness severity led to increased testing. Patients tested for C-diff had longer LOS likely due to increased testing during lengthier admissions; those not tested had shorter time to readmission perhaps indicating broader barriers to care. This highlights the pivotal role of GI consultants and the need to promote non-GI provider awareness for C-diff testing to improve IBD patient care.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Danielle Garfunkel, MD1, Daniel Alvarez, MD2, George G.. Papadopoulos, MD3, Hamza Sadhra, MD3, Melissa Fazzari, PhD, MS4, Ruby Greywoode, MD, MS4, Thomas Ullman, MD4. P0229 - Impact of Patient and Provider Variability on Compliance With C. difficile Testing for IBD Flares, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Montefiore Medical Center, Scarsdale, NY; 2Montefiore Medical Center/Albert Einstein College of Medicine, Bronx, NY; 3Montefiore Medical Center, Bronx, NY; 4Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, NY
Introduction: Over three decades, the incidence of Clostridioides difficile (C-diff) in inflammatory bowel disease (IBD) patients has risen dramatically. Though guidelines advise testing all patients with IBD flares for C-diff, there is little data on compliance with this recommendation. This study investigates C-diff testing compliance rates, factors affecting testing and impact of testing in IBD flares on length of stay (LOS) and readmissions.
Methods: We conducted a retrospective cohort study at a tertiary academic medical center of adults admitted for IBD flares with documented diarrhea from 3/2019-3/2021. We excluded patients who left the emergency department (ED) against medical advice. Demographic and clinical data were gathered by chart review. Primary outcome was C-diff testing rates. Secondary outcomes included time to testing. Admissions from 3/2019-3/2020 were compared to 3/2020-3/2021 to evaluate the COVID pandemic effect. We employed chi-square and Wilcoxon Mann-Whitney tests to examine associations and multivariate Poisson regression model to adjust for confounders.
Results: Of 802 admissions, 195 (24%) were included. The mean age was 38 years with 55% males, 55% of Hispanic ethnicity and 53% with ulcerative colitis (Table 1). 83% of cases were tested for C-diff with a 7% positivity rate. 71% were sent in the inpatient setting rather than the ED and 66% were sent before day 2 of hospitalization. Of those not tested, 30% had resolved diarrhea and 12% were canceled by lab (Fig. 1). Factors associated with increased testing included gastroenterology consultation (85% vs 63%, p=0.02), higher heart rate (98 vs 87, p=0.02), and positive SIRS criteria (91% vs 79%, p=0.04). During the COVID pandemic, ED testing rates declined (38% vs 18%, p=0.01). After adjusting for confounders, expected LOS was 54% longer among those tested than not tested (p< 0.0001). Those tested tended to have longer time to readmission (104d vs 45d, p=0.19).
Discussion: At our hospital, C-diff testing rates for IBD flares were relatively high, though most tests were delayed until inpatient admission. Gastroenterology consultation and patient illness severity led to increased testing. Patients tested for C-diff had longer LOS likely due to increased testing during lengthier admissions; those not tested had shorter time to readmission perhaps indicating broader barriers to care. This highlights the pivotal role of GI consultants and the need to promote non-GI provider awareness for C-diff testing to improve IBD patient care.

Figure: Figure 1: Reasons Why C-diff Tests Were Not Sent
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Danielle Garfunkel indicated no relevant financial relationships.
Daniel Alvarez indicated no relevant financial relationships.
George Papadopoulos indicated no relevant financial relationships.
Hamza Sadhra indicated no relevant financial relationships.
Melissa Fazzari indicated no relevant financial relationships.
Ruby Greywoode: Janssen – Grant/Research Support.
Thomas Ullman: BMS – Consultant. Pfizer – Advisor or Review Panel Member, Grant/Research Support.
Danielle Garfunkel, MD1, Daniel Alvarez, MD2, George G.. Papadopoulos, MD3, Hamza Sadhra, MD3, Melissa Fazzari, PhD, MS4, Ruby Greywoode, MD, MS4, Thomas Ullman, MD4. P0229 - Impact of Patient and Provider Variability on Compliance With C. difficile Testing for IBD Flares, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.

