Sunday Poster Session
Category: Colon
P0238 - Real World Outcomes of Fecal Microbiota-Based Therapies to Prevent Recurrent Clostridioides Difficile Infections up to 24 Weeks
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E
Has Audio

Larry Nguyen, MD
NYU Grossman School of Medicine
New York, NY
Presenting Author(s)
Larry Nguyen, MD1, Jessica R. Allegretti, MD, MPH, FACG2, Grace Hardwick, 3, Jordan Axelrad, MD, MPH1
1NYU Grossman School of Medicine, New York, NY; 2Brigham and Women’s Hospital, Harvard Medical School, Boston, MA; 3Brigham and Women’s Hospital, Boston, MA
Introduction: Microbial therapies are emerging as effective strategies for preventing recurrent Clostridioides difficile infection (rCDI). Fecal microbiota, live-jslm, the first rectally administered microbiota live product, and Fecal microbiota spores, live-brpk, the first orally administered fecal microbiota product were recently FDA approved for the prevention of rCDI. Here, we report a real-world efficacy analyzes of these novel microbial therapies in preventing rCDI.
Methods: Adults with rCDI treated with Fecal microbiota, live-jslm, Fecal microbiota spores, live-brpk, or conventional fecal microbiota transplant (FMT) were identified between October 2014 and May 2024. All Fecal microbiota, live-jslm and FMT were administered via colonoscopy. Recurrence of CDI was defined by relapse of diarrheal symptoms, confirmatory stool testing, and subsequent standard-of-care antibiotic treatment. Treatment success rate and clinical cure rate was defined as absence of rCDI for 8- and 24-weeks post-treatment, respectfully. Efficacy was stratified based on age, standard of care antibiotic, duration of prior antibiotic treatment, number of prior CDI episodes and presence of inflammatory bowel disease (IBD).
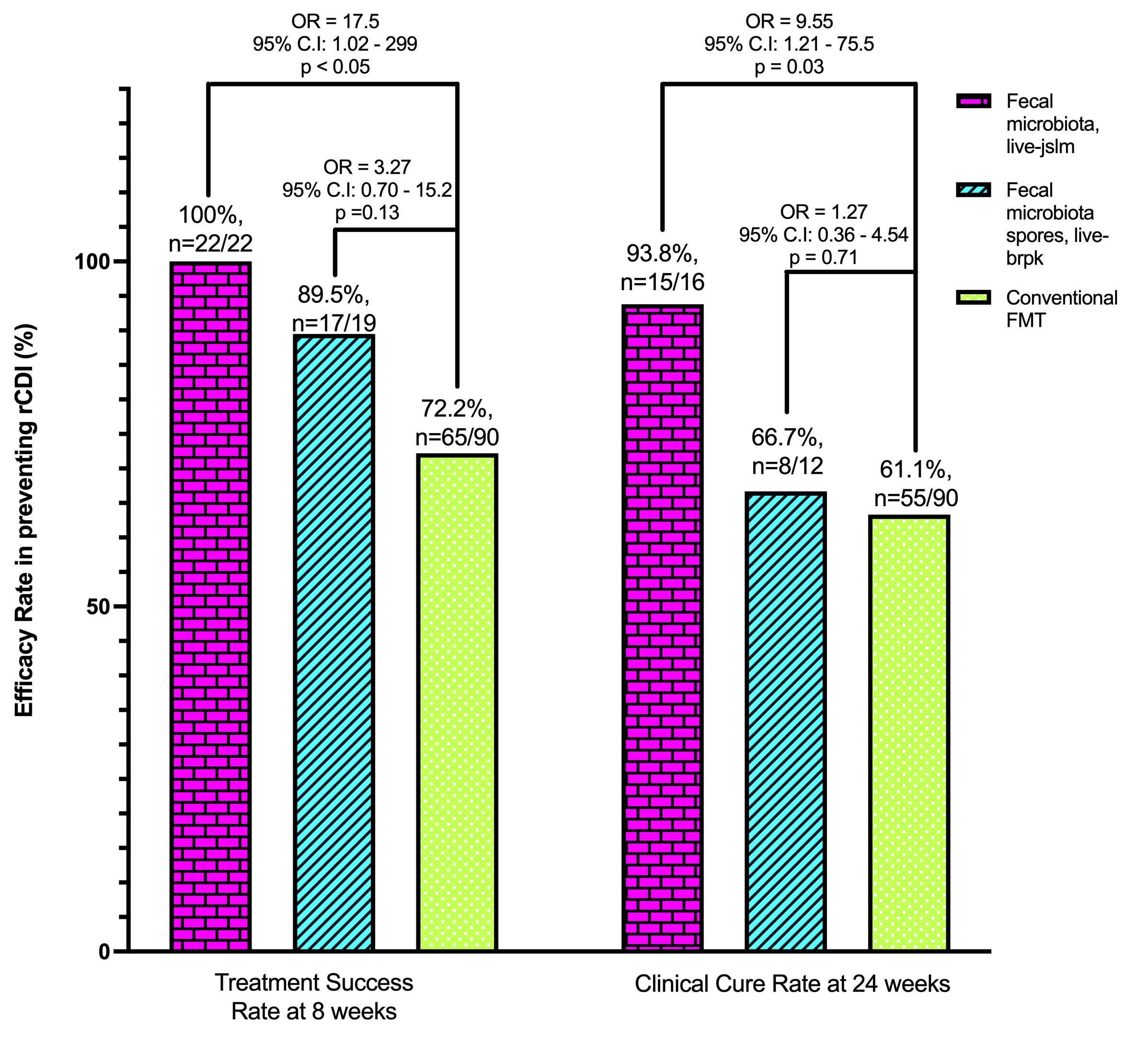
Results: We identified 22 patients who received Fecal microbiota, live-jslm, 19 received Fecal microbiota spores, live-brpk, and 90 received conventional FMT for rCDI prevention. Treatment success in preventing rCDI at week 8 was 100%, 89.5%, and 72.2% for Fecal microbiota, live-jslm, Fecal microbiota spores, live-brpk, and conventional FMT, respectively (Figure; p < 0.05, p = 0.13). Clinical cure rate in preventing rCDI at week 24 was 93.8%, 66.7%, and 61.1% for Fecal microbiota, live-jslm, Fecal microbiota spores, live-brpk, and conventional FMT, respectively (p = 0.03, p = 0.71). Stratifying efficacy by all covariates up to 24 weeks demonstrated the equivalent efficacy of Fecal microbiota, live-jslm and Fecal microbiota spores, live-brpk compared to conventional FMT (Table). No serious treatment-related adverse events were reported in this study.
Discussion: Our real-world data demonstrates the efficacy of these novel fecal microbiota-based therapies in preventing rCDI. Fecal microbiota, live-jslm demonstrated superior overall efficacy compared to conventional FMT in preventing rCDI up to 24 weeks post-treatment. Further analysis with larger sample size and combination with other therapies are required to determine the most efficient strategy to prevent rCDI.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Larry Nguyen, MD1, Jessica R. Allegretti, MD, MPH, FACG2, Grace Hardwick, 3, Jordan Axelrad, MD, MPH1. P0238 - Real World Outcomes of Fecal Microbiota-Based Therapies to Prevent Recurrent <i>Clostridioides Difficile</i> Infections up to 24 Weeks, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1NYU Grossman School of Medicine, New York, NY; 2Brigham and Women’s Hospital, Harvard Medical School, Boston, MA; 3Brigham and Women’s Hospital, Boston, MA
Introduction: Microbial therapies are emerging as effective strategies for preventing recurrent Clostridioides difficile infection (rCDI). Fecal microbiota, live-jslm, the first rectally administered microbiota live product, and Fecal microbiota spores, live-brpk, the first orally administered fecal microbiota product were recently FDA approved for the prevention of rCDI. Here, we report a real-world efficacy analyzes of these novel microbial therapies in preventing rCDI.
Methods: Adults with rCDI treated with Fecal microbiota, live-jslm, Fecal microbiota spores, live-brpk, or conventional fecal microbiota transplant (FMT) were identified between October 2014 and May 2024. All Fecal microbiota, live-jslm and FMT were administered via colonoscopy. Recurrence of CDI was defined by relapse of diarrheal symptoms, confirmatory stool testing, and subsequent standard-of-care antibiotic treatment. Treatment success rate and clinical cure rate was defined as absence of rCDI for 8- and 24-weeks post-treatment, respectfully. Efficacy was stratified based on age, standard of care antibiotic, duration of prior antibiotic treatment, number of prior CDI episodes and presence of inflammatory bowel disease (IBD).
Results: We identified 22 patients who received Fecal microbiota, live-jslm, 19 received Fecal microbiota spores, live-brpk, and 90 received conventional FMT for rCDI prevention. Treatment success in preventing rCDI at week 8 was 100%, 89.5%, and 72.2% for Fecal microbiota, live-jslm, Fecal microbiota spores, live-brpk, and conventional FMT, respectively (Figure; p < 0.05, p = 0.13). Clinical cure rate in preventing rCDI at week 24 was 93.8%, 66.7%, and 61.1% for Fecal microbiota, live-jslm, Fecal microbiota spores, live-brpk, and conventional FMT, respectively (p = 0.03, p = 0.71). Stratifying efficacy by all covariates up to 24 weeks demonstrated the equivalent efficacy of Fecal microbiota, live-jslm and Fecal microbiota spores, live-brpk compared to conventional FMT (Table). No serious treatment-related adverse events were reported in this study.
Discussion: Our real-world data demonstrates the efficacy of these novel fecal microbiota-based therapies in preventing rCDI. Fecal microbiota, live-jslm demonstrated superior overall efficacy compared to conventional FMT in preventing rCDI up to 24 weeks post-treatment. Further analysis with larger sample size and combination with other therapies are required to determine the most efficient strategy to prevent rCDI.

Figure: Overall Efficacy of Fecal microbiota, live-jslm and Fecal microbiota spores, live-brpk Compared to Conventional FMT in preventing rCDI at 8-and 24-weeks.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Larry Nguyen indicated no relevant financial relationships.
Jessica R. Allegretti: Abbvie – Consultant, Speakers Bureau. Artugen – Consultant. Bristol Myers Squibb – Consultant, Speakers Bureau. Ferring – Consultant. Finch Therapeutics – Consultant. Janssen – Consultant. Merck – Grant/Research Support. Pfizer – Consultant. Seres – Consultant.
Grace Hardwick indicated no relevant financial relationships.
Jordan Axelrad: Abbvie – Consultant. Adiso – Consultant. Biomerieux – Consultant. BMS – Consultant. Fresenius – Consultant. Genentech – Grant/Research Support. Janssen – Consultant. Pfizer – Consultant.
Larry Nguyen, MD1, Jessica R. Allegretti, MD, MPH, FACG2, Grace Hardwick, 3, Jordan Axelrad, MD, MPH1. P0238 - Real World Outcomes of Fecal Microbiota-Based Therapies to Prevent Recurrent <i>Clostridioides Difficile</i> Infections up to 24 Weeks, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
